Everything ECMO 043: Persistent and refractory hypoxaemia on VV-ECMO – Part 1
Author: Dr Alastair Brown
Peer reviewers: Dr Aidan Burrell, Dr Arne Diehl, A/Prof Chris Nickson
A 32-year-old man presented to hospital with severe streptococcal community acquired pneumonia (CAP) and respiratory distress. His initial oxygen saturation (SpO2) on room air was 71% and only improved with high-flow oxygen to 83%. The patient rapidly proceeded to intubation but remained hypoxaemic. VV-ECMO support was commenced via a right 23Fr femoral access cannula and left 21Fr femoral return cannula.
The patient was initially well supported on ECMO with a blood flow of 3.8 L/min, fresh gas flow of 8 L/min and peripheral SpO2 96%.
The following day the patient was taken for a CT scan. During transport the Sp02 is noted to drop abruptly to 72%. There were no alarms on the ventilator or ECMO circuit. Chest radiograph is shown in Figure 1.
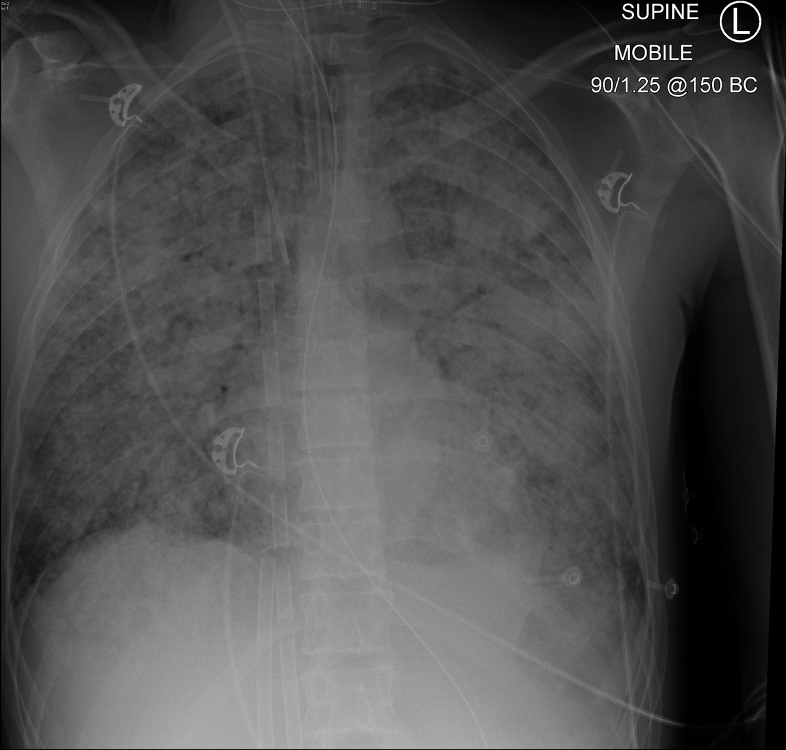
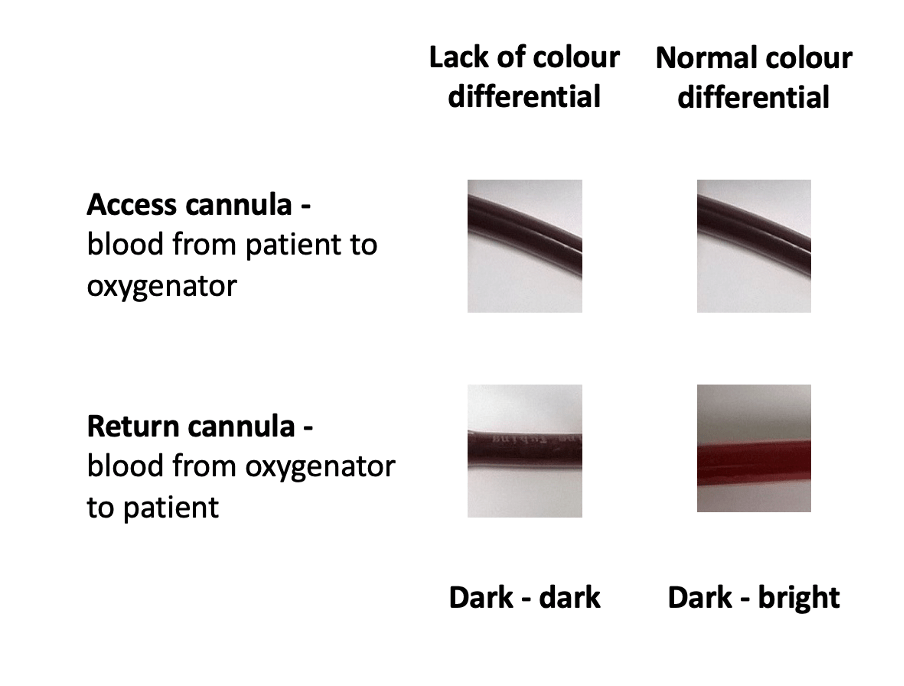
Q1. The ECMO cannula tubing looks as shown below (Figure 2). What is the most important circuit component to check next?
Check for oxygen supply failure – is oxygen is connected and flowing to the ECMO console?
Disconnection of oxygen tubing during transport is an emergency. There is no alarm (!) on the ECMO circuit to highlight a lack of oxygen delivery to the membrane.
Signs of oxygen supply failure include:
- Lack of colour differential between access and return cannula (see Figure 3 below)
- Patient desaturation (falling SpO2 – a late sign)
- Reduced access saturation, displayed on ECMO console (this may generate an alarm depending on the setting, typical if they fall below 40% – also a late sign of oxygen supply failure)
Over the next few days, the patient’s ventilation continues to deteriorate and compliance decreases to 4 mL/cmH2O and SpO2 ranges from 85-88%. Titration of positive end expiratory pressure (PEEP) and achieving a negative fluid balance through diuresis the patient failed to improve the situation.
Q2. What are options for ongoing patient management?
- Increase ECMO flow: This is a good option if there is no access insufficiency and recirculation not a clinical problem. In general, maintain the pump speed < 4000 rpm, although in some circumstances this may need to be increased.
- Tolerate SpO2 >85%: A key consideration during VV ECMO is oxygen delivery. If patient saturations are low but oxygen delivery appears adequate (consider Hb, oxygen saturation, and oxygen extraction and ensure low lactate and no related new organ failure) it is often reasonable to reduce the SpO2 target rather than undertake additional interventions.
- Prone positioning: While not a common intervention patients can be prone positioned during VV-ECMO. This intervention has been shown to reduce mortality in patients with moderate to severe ARDS not receiving ECMO and was suggested to be beneficial in a recent meta-analysis of ECMO patients1,2. It is currently being further evaluated in ECMO patients3.
- Transfuse to higher haemoglobin target: Increasing Hb concentration will increase oxygen content and oxygen delivery. The extraction ratio will also decrease and mixed venous saturation will increase. As a result less oxygen will need to be added to the blood to achieve a normal saturation. This is a common practice in some ECMO centres4,5, however the risk/benefit has not been fully assessed. It should be noted that in non-ecmo cohorts (also not hypoxic) that conservative transfusion target have been shown to be equivalent or superior to a liberal transfusion targets6,7.
- Start Nitric Oxide: Nitric Oxide may improve V:Q matching in the lung and improve saturation but this has not been shown to reduce mortality8. The physiologic effect and the added benefit are likely to be negligible in ECMO patients when the tidal volumes are very low.
3 days later, the same patient has developed worsening hypoxaemia. His chest radiograph is unchanged (see image). ECMO flow has been increased to 4.5 L/min with increasing issues with access insufficiency. The SpO2 is 86% and pre-oxygenator SO2 is 50%. The patient has also developed an increased noradrenaline requirement up to 30 mcg/min and has been started on vasopressin 2 units/h.
Q3. What is the likely cause of the worsening hypoxaemia in this patient’s case?
The likely cause in this patient is increased cardiac output in the setting of septic shock.
Other potential causes of hypoxaemia also need to be considered and excluded (see Everything ECMO 001 for a stepwise approach to hypoxaemia on VV-ECMO). Also note that the unchanged cannulae positions on the chest radiograph and the low pre-oxygenator SO2 exclude recirculation as a clinically significant issue.
Increased cardiac output contributes to hypoxaemia on VV-ECMO as follows:
- During severe respiratory failure the shunt fraction is high.
- Blood passing through the lungs without passing through the ECMO membrane will not be oxygenated effectively.
- As the patient’s cardiac output increases the proportion of the cardiac output passing through the ECMO membrane will decrease resulting in decreased patient saturations (SpO2 and SaO2).
Q4. How can this be managed?
Possible solutions to this issue are:
- Ensure current ECMO cannula position and oxygenator function is optimal.
- Treat underlying cause of increased cardiac output: treat sepsis and provide adequate doses of vasopressor4,5.
- Convert to high flow configuration: a second access cannula is added to achieve higher flow for a given RPM a radiographic example is shown below4,5. With a well-placed primary access cannula the effect is usually rather small and may add 0.5- 1 L/min blood flow. In the setting of high cardiac output it might be challenging to substantially improve oxygen saturation in this manner.
- Targeted temperature management: lowering patient temperature may decrease oxygen demand and improve oxygen saturations. However, the benefits of this approach are unproven, it has been described in a case report9.
- Control cardiac output with beta blockers: The rationale for this strategy is to increase the ratio of ECMO flow (QECMO) to native cardiac output, due to the negative chrontropic and negative inotropic effects of beta blockers. As a result a higher proportion of the cardiac output will be oxygenated by the membrane. There are a few reports of this strategy in the literature and it appears to increase SpO210,11. However the concern remains that while SpO2 may increase the reduction in cardiac output may counteract an increase in oxygen content and result in no improvement in oxygen delivery12. A further factor to consider is that beta-blockers may also reduce oxygen consumption13, and there are data suggesting titratable beta blockers may reduce mortality in sepsis14,15. Further studies are required before this treatment is recommended for hypoxaemia during VV-ECMO.
References
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159-2168. doi:10.1056/NEJMoa1214103 https://pubmed.ncbi.nlm.nih.gov/23688302/
- Papazian L, Schmidt M, Hajage D, et al. Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med. 2022;48(3):270-280. doi:10.1007/s00134-021-06604-x https://pubmed.ncbi.nlm.nih.gov/35037993/
- PRONing to Facilitate Weaning From ECMO in Patients With Refractory Acute Respiratory Distress Syndrome (PRONECMO) https://clinicaltrials.gov/ct2/show/NCT04607551
- Messai E, Bouguerra A, Guarracino F, Bonacchi M. Low Blood Arterial Oxygenation During Venovenous Extracorporeal Membrane Oxygenation: Proposal for a Rational Algorithm-Based Management. J Intensive Care Med. 2016;31(8):553-560. doi:10.1177/0885066616649134 https://pubmed.ncbi.nlm.nih.gov/27271548/
- Montisci A, Maj G, Zangrillo A, Winterton D, Pappalardo F. Management of refractory hypoxemia during venovenous extracorporeal membrane oxygenation for ARDS. ASAIO J. 2015;61(3):227-236. doi:10.1097/MAT.0000000000000207 https://pubmed.ncbi.nlm.nih.gov/25923575/
- Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381-1391. doi:10.1056/NEJMoa1406617 https://pubmed.ncbi.nlm.nih.gov/25270275/
- Hughes T, Zhang D, Nair P, Buscher H. A Systematic Literature Review of Packed Red Cell Transfusion Usage in Adult Extracorporeal Membrane Oxygenation. Membranes (Basel). 2021;11(4):251. Published 2021 Mar 30. doi:10.3390/membranes11040251 https://pubmed.ncbi.nlm.nih.gov/33808419/
- Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;2016(6):CD002787. Published 2016 Jun 27. doi:10.1002/14651858.CD002787.pub3 https://pubmed.ncbi.nlm.nih.gov/27347773/
- Patel B, Arcaro M, Chatterjee S. Bedside troubleshooting during venovenous extracorporeal membrane oxygenation (ECMO). J Thorac Dis. 2019;11(Suppl 14):S1698-S1707. doi:10.21037/jtd.2019.04.81 https://pubmed.ncbi.nlm.nih.gov/31632747/
- Guarracino F, Zangrillo A, Ruggeri L, et al. β-Blockers to optimize peripheral oxygenation during extracorporeal membrane oxygenation: a case series. J Cardiothorac Vasc Anesth. 2012;26(1):58-63. doi:10.1053/j.jvca.2011.05.013 https://pubmed.ncbi.nlm.nih.gov/21764329/
- Bunge, J. J. H. et al. Safety and efficacy of beta-blockers to improve oxygenation in patients on veno-venous ECMO. J Crit Care 53, 248–252 (2019). https://pubmed.ncbi.nlm.nih.gov/21764329/
- Droogh JM, Oude Lansink A, Renes MH, Metz E, Nijsten MW. In veno-venous ECMO oxygen delivery should be the focus. J Crit Care. 2019;54:76. doi:10.1016/j.jcrc.2019.07.016 https://pubmed.ncbi.nlm.nih.gov/31382218/
- Cocchi MN, Dargin J, Chase M, et al. Esmolol to Treat the Hemodynamic Effects of Septic Shock: A Randomized Controlled Trial. Shock. 2022;57(4):508-517. doi:10.1097/SHK.0000000000001905 https://pubmed.ncbi.nlm.nih.gov/35066509/
- Gadallah, R.R., Aboseif, E.M.K., Ibrahim, D.A. et al. Evaluation of the safety and efficacy of beta blockers in septic patients: a randomized control trial. Ain-Shams J Anesthesiol 12, 57 (2020). https://doi.org/10.1186/s42077-020-00107-5 https://asja.springeropen.com/articles/10.1186/s42077-020-00107-5
- Morelli A, Singer M, Ranieri VM, et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med. 2016;42(10):1528-1534. doi:10.1007/s00134-016-4351-2 https://pubmed.ncbi.nlm.nih.gov/27101380/

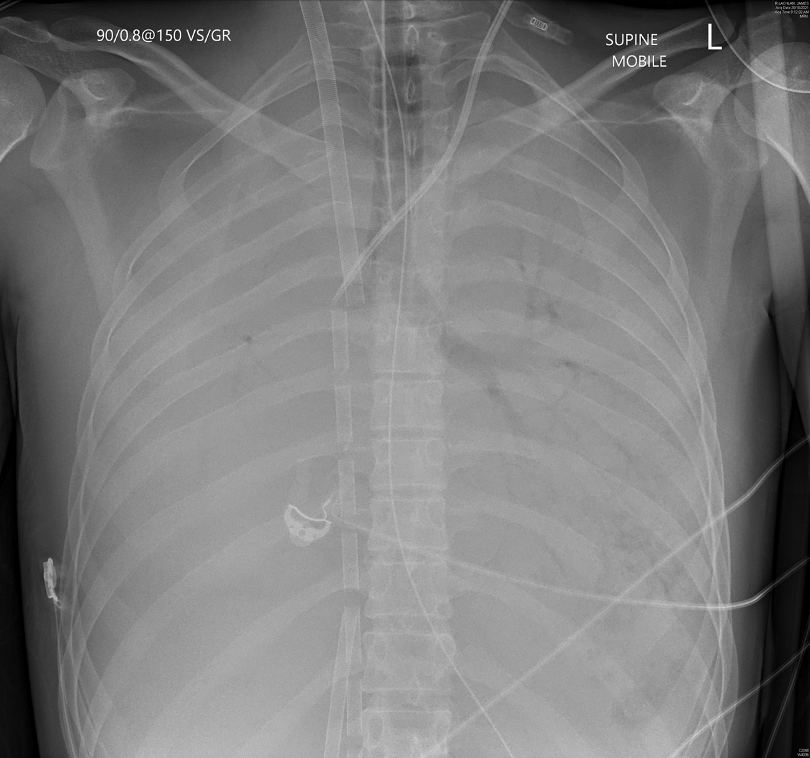
Shouldn’t the drainage be lower than return in Fem-Fem VV? On cxr in Ecmo # 43
Our approach to to V-V ECMO cannula placement is as follows (excerpt from https://ecmo.icu/procedures-percutaneous-ecmo-cannulation/?kw=cannula+placement#pageautoanchor-11 – the link also has nice labelled CXR showing cannula position)
Venous access
Ultrasound-guided placement into the high RA or SVC. Using a multistage cannula, it is important to note that most blood enters the cannula via the most proximal (first) hole. It is essential that this portion of the cannula is sited as close to the cavo-atrial junction as possible. As a result, the tip of the multistage cannula will be beyond the RA when optimally placed (see below).
Marked reductions to PEEP following ECMO placement may result in a lower (suboptimal) cannula position after cannulation due to relative lung deflation. It is preferred and safe to place the cannula relatively high rather than too low initially.
Venous return
The single-stage return cannula is guided into the right atrium generally with a separation between access (proximal, active draining holes of the access cannula) and return cannula of at least 5 cm beyond the proximal hole of the multistage cannula to prevent clinically significant recirculation in the circuit (see image below). This refers to the radio-opaque part of the venous return cannula. There is an additional 4 cm length of a perforated tip that is not visible on the X-ray.