Author: Lewis McLean
Peer reviewers: Li Tan, Aidan Burrell, Trent Hartshorne, Chris Nickson
Everything ECMO 030
A 45-year-old patient is brought into the intensive care unit (ICU) after an out-of-hospital cardiac arrest (OOHCA). The initial rhythm was ventricular fibrillation (VF) and the now has return of spontaneous circulation (ROSC) but remains shocked. The vasopressor doses are going up and up………
Q1. What is the role of ultrasound in extra-corporeal membrane oxygenation (ECMO) management?
Ultrasound has 3 roles in ECMO management (aka extracorporeal life support (ECLS)):
- Starting (initiation)
- Stopping (weaning)
- Troubleshooting (stable and unstable)
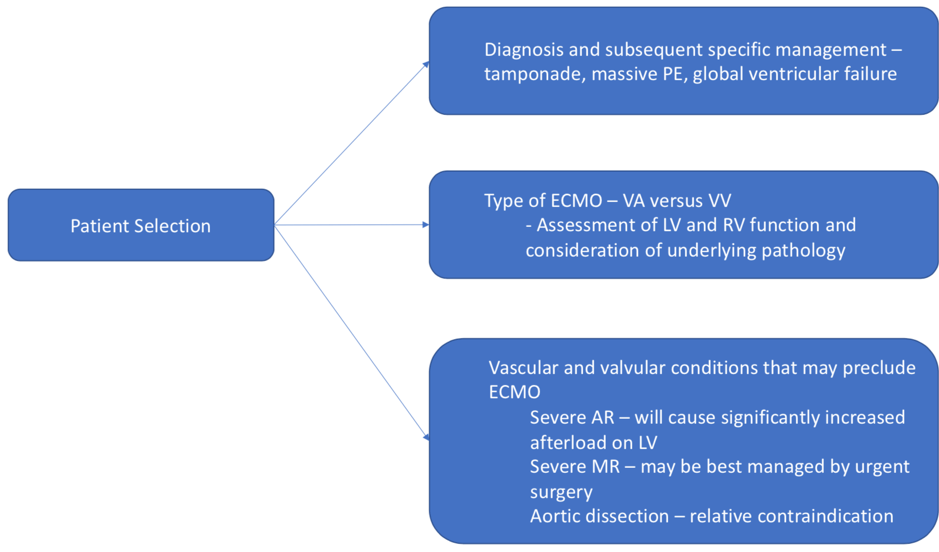
Q2. How is ultrasound used in the initiation of ECMO management, in general?
Ultrasound is used in initiation of ECLS in three ways1:
In ECPR, not all of these options are necessarily relevant or feasible. Ultrasound in ECPR can be used for:
- Patient selection (e.g. exclude dissection, haemorrhage from prolonged CPR)
- Vascular access and confirmation of guidewire and cannula position
- Assess for complications post-ECMO initiation (e.g. aortic regurgitation, haemorrhage from prolonged CPR)
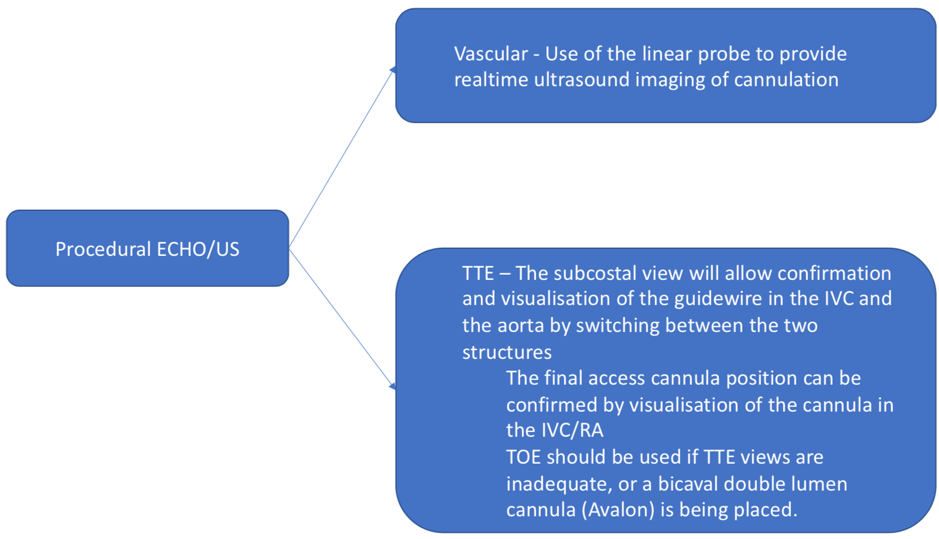
Q3. What are these views, and how are they used?
This is a transoesphageal echo (TOE) bicaval view. The mid oesophageal bicaval view (transducer angle 90 to 110 o) is used to confirm venous placement of the access guidewire for V-A and V-V ECMO, and to guide positioning of the cannula.
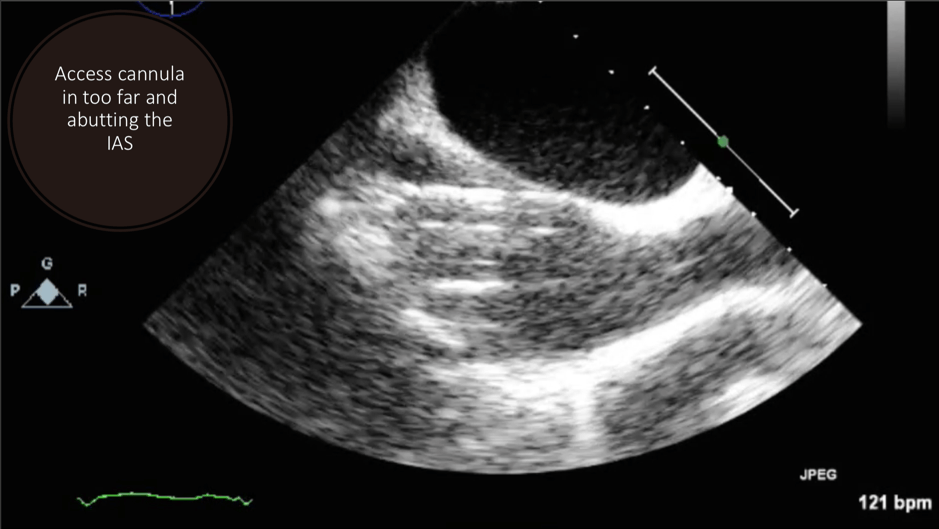
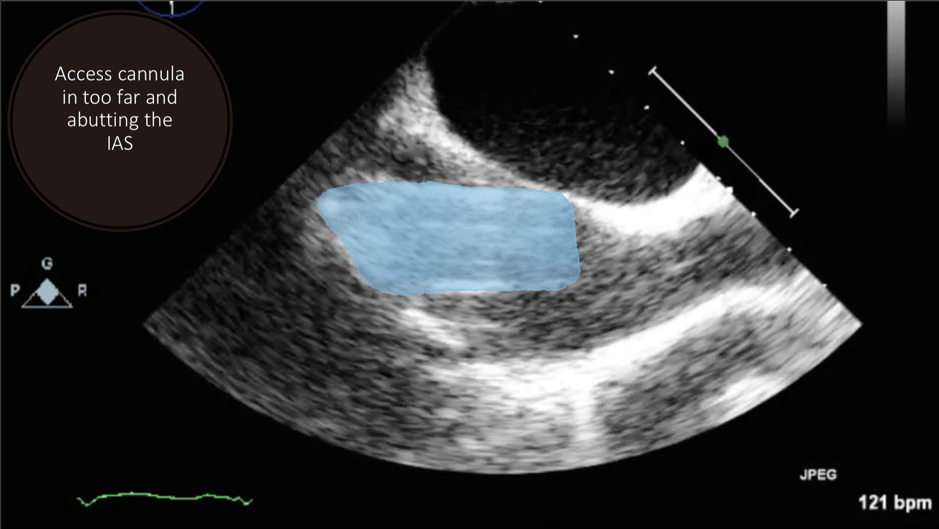
Ideally the access cannula in femoral V-A ECMO should be sited slightly into the right atrium (RA). In V-V ECMO, it is the return cannula which is sited at this point.
The correctly positioned guide wire should bypass the hepatic vein. The cannula should NOT traverse the RA into the superior vena cava (SVC) – careful scanning is required to demonstrate the cannula tip to avoid this.
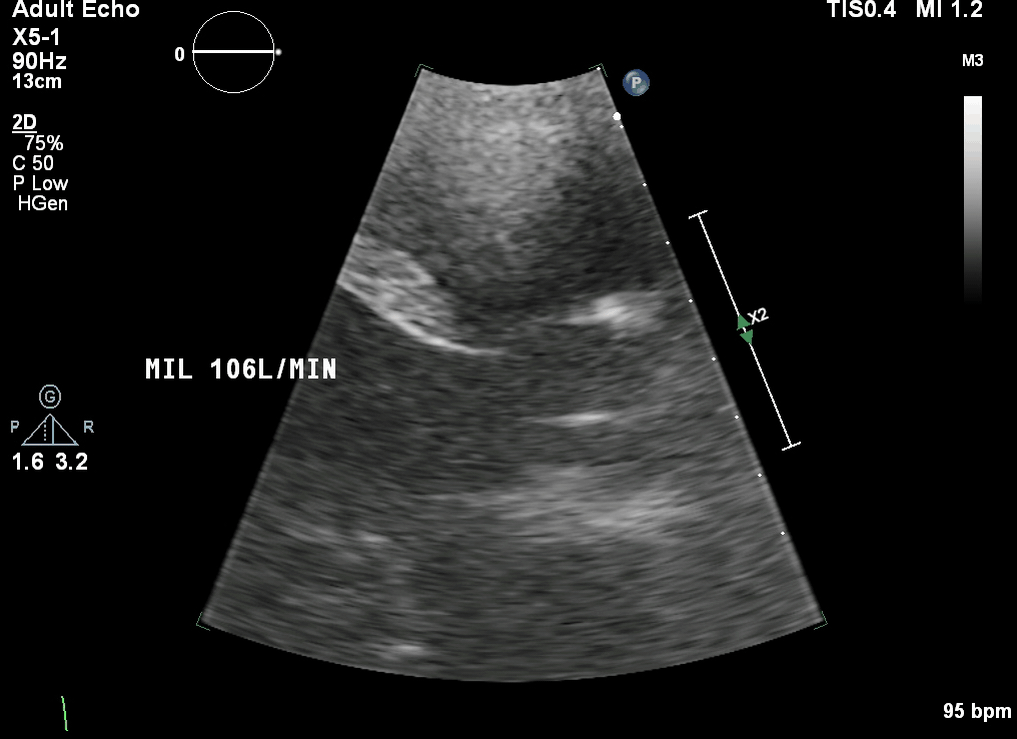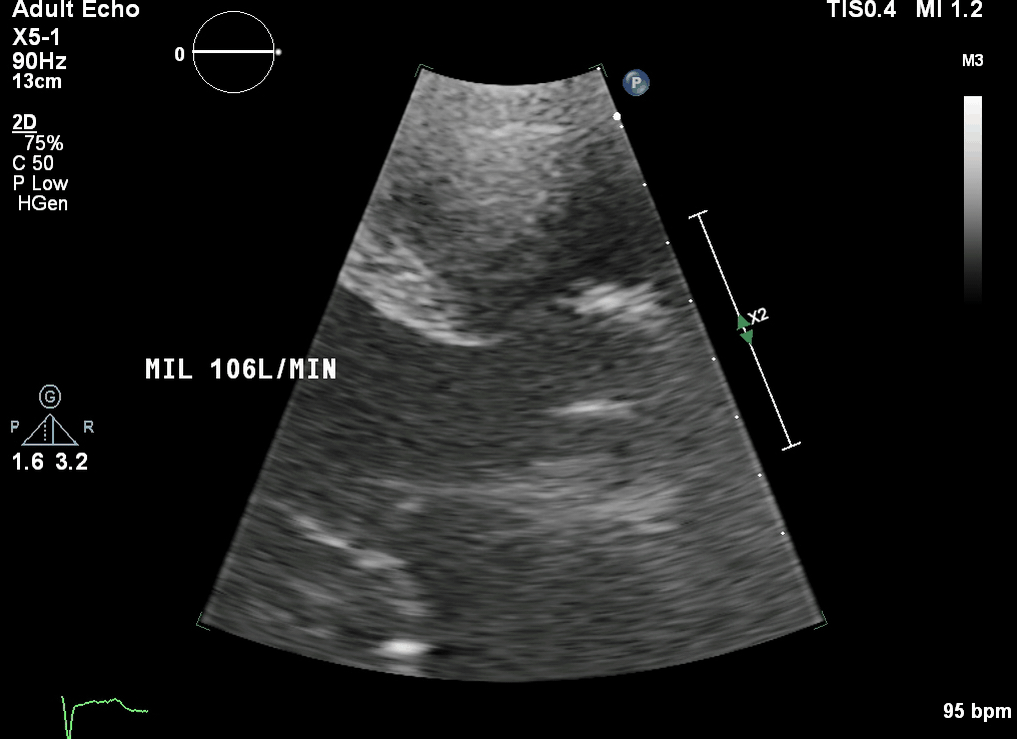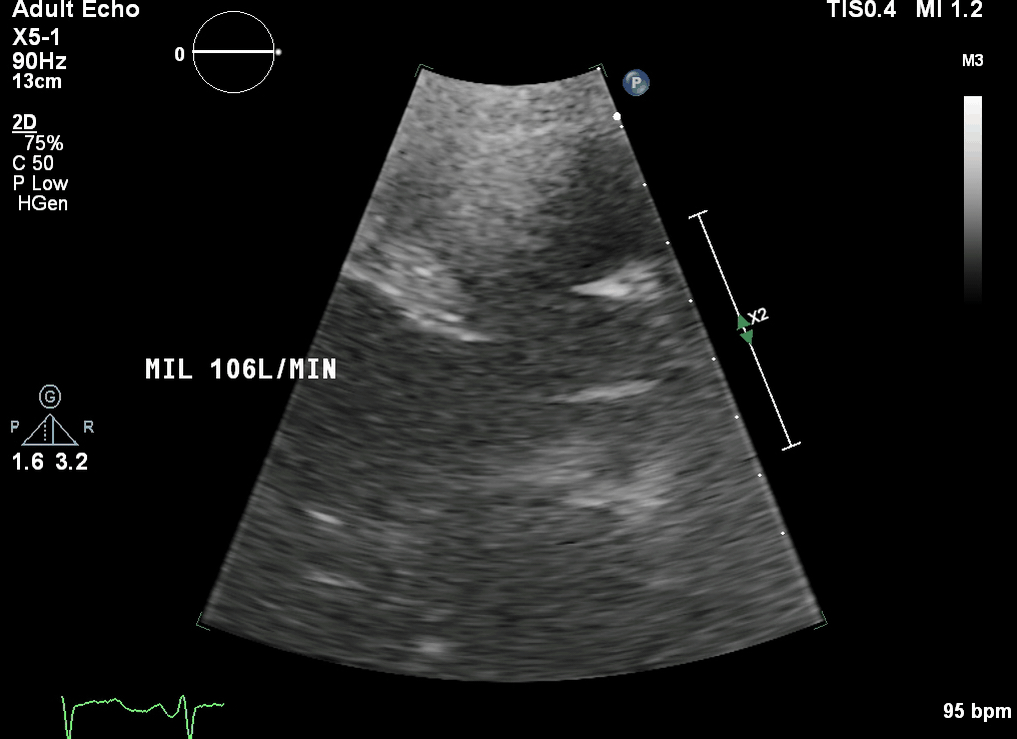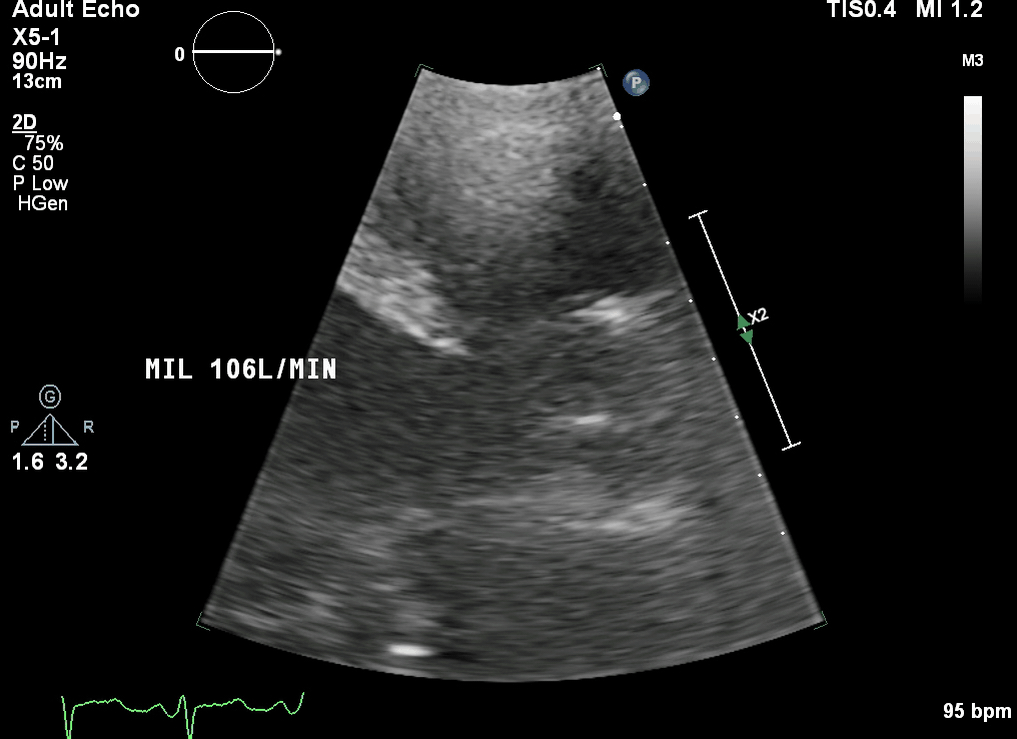
This is a transthoracic echo (TTE) subcostal short axis view demonstrating the inferior vena cava (IVC) and RA. This is the usual view obtained during cannulation to guide wire and cannula insertion, unless there are poor subcostal views, in which case TOE is used. It is used in a similar manner to the TOE bicaval view to confirm correct guidewire and cannula placement. As the SVC is not well visualised, the tip of the cannula must be seen and confirmed in the RA/IVC.
This is a TTE subcostal short axis view demonstrating the IVC and RA. This is the usual view obtained during cannulation to guide wire and cannula insertion, unless there are poor subcostal views, in which case TOE is used. It is used in a similar manner to the TOE bicaval view to confirm correct guidewire and cannula placement. As the SVC is not well visualised, the tip of the cannula must be seen and confirmed in the RA/IVC.
See Everything ECMO 029 for more on this topic.
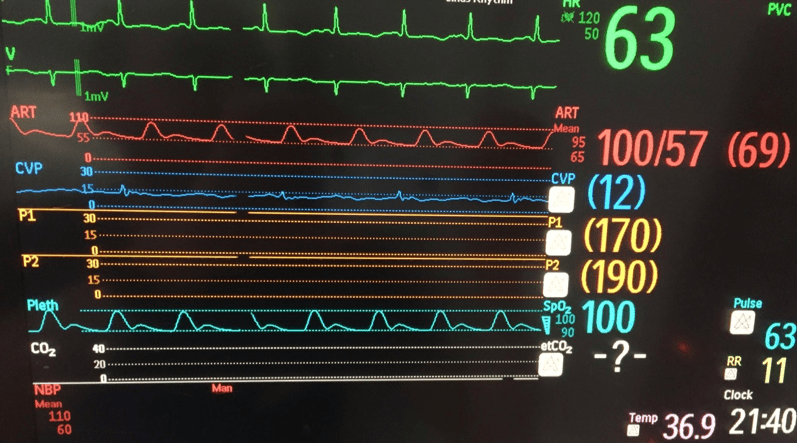
Q4. The patient is stabilised on V-A ECMO and sent to ICU after PCI of an occluded LAD lesion. 2 days later, there is a sudden threefold increase in vasopressor requirement. The arterial line trace has gone from this:
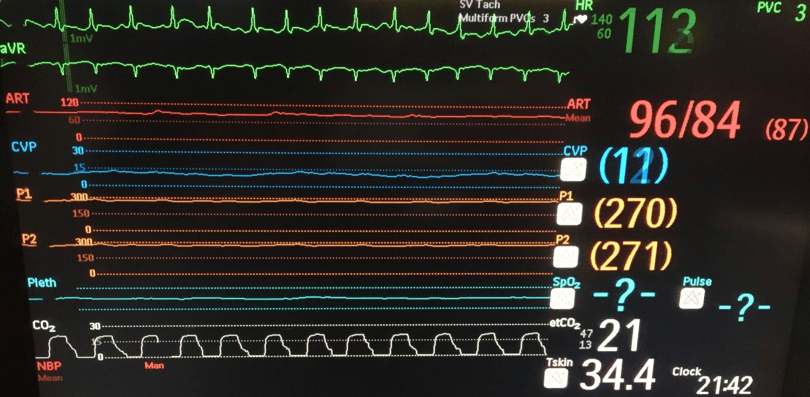
To this:
What has occurred and what are the potential causes?
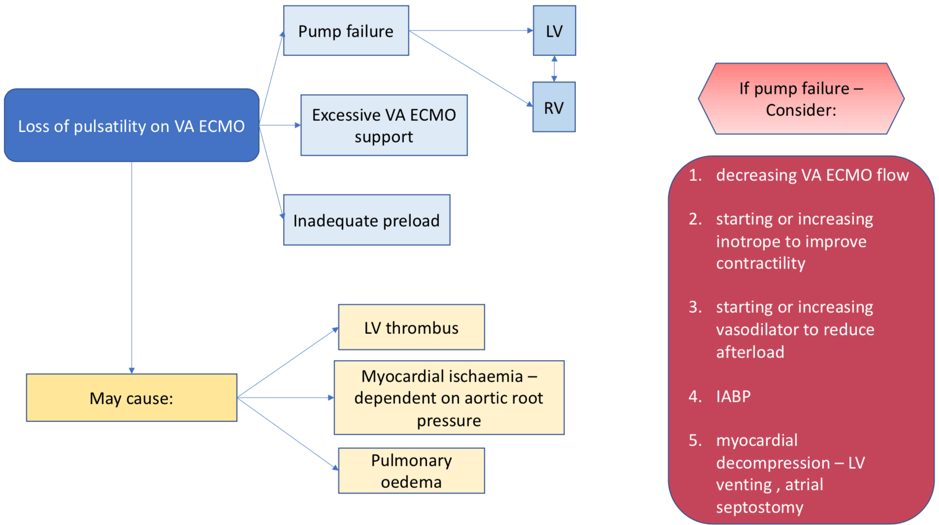
There is a loss of pulsatility.
The three major causes in ECMO are:
- Pump failure
- Hypovolaemia/bleeding
- Cardiac tamponade
Q5. How can ultrasound be used in differentiating these conditions?
Echocardiography can be used to assess the hemodynamic state and detect complications in this situation, looking specifically for2-4:
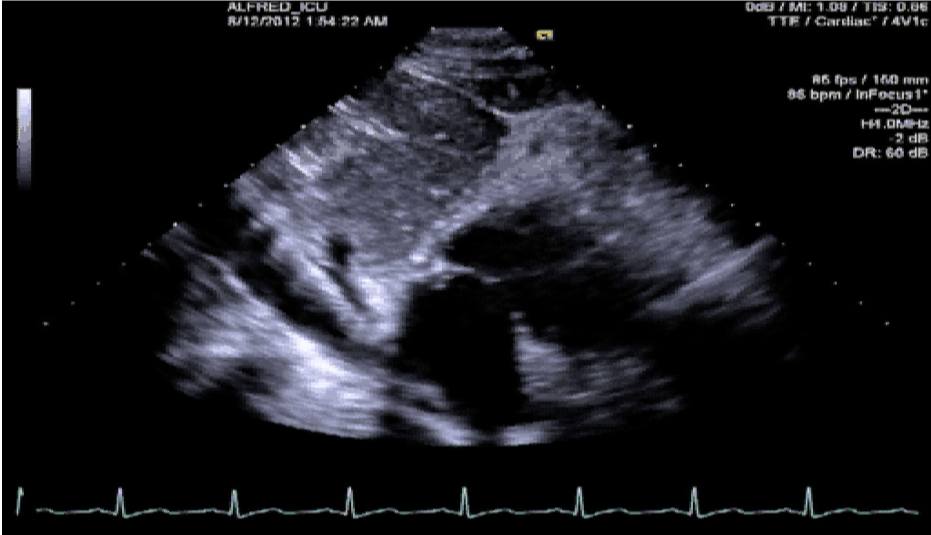
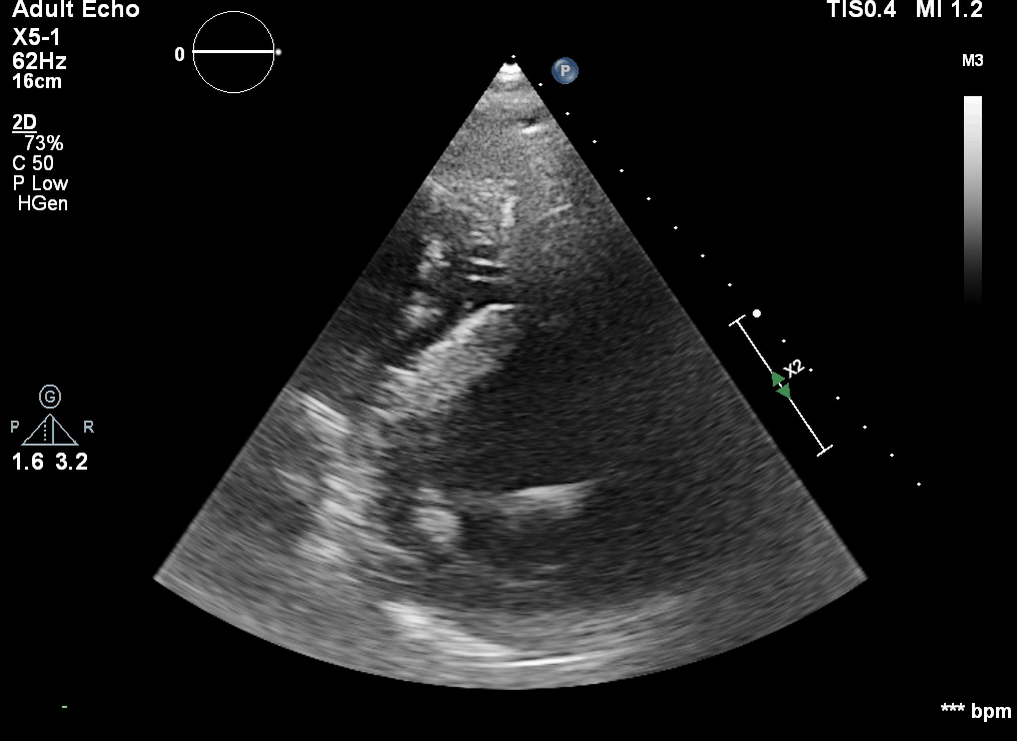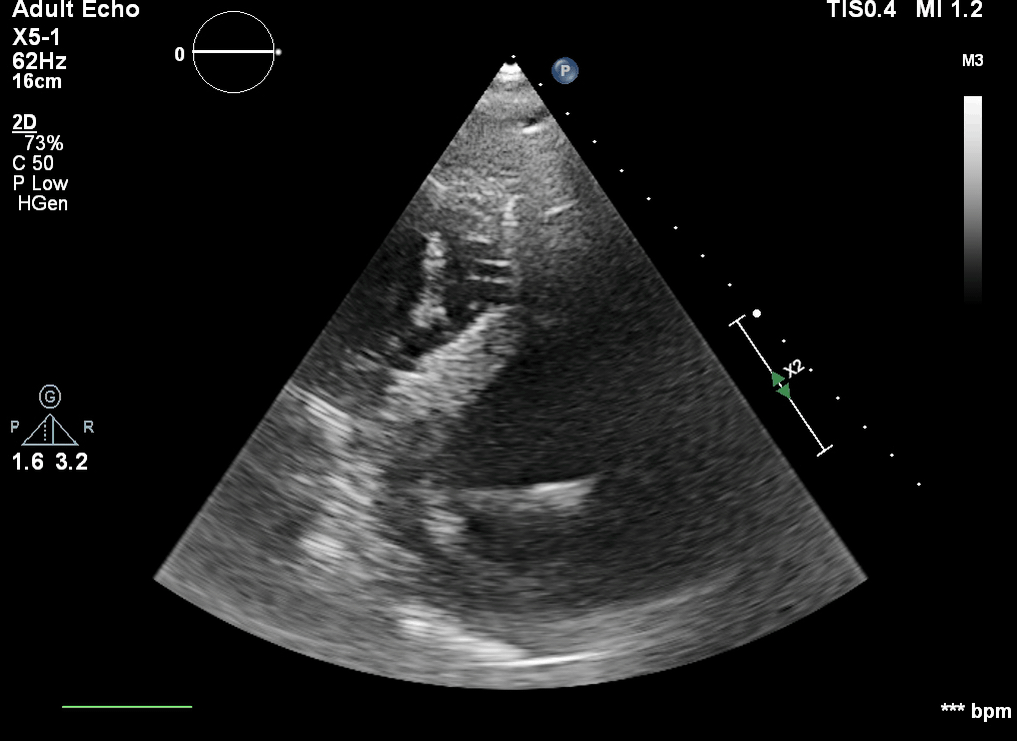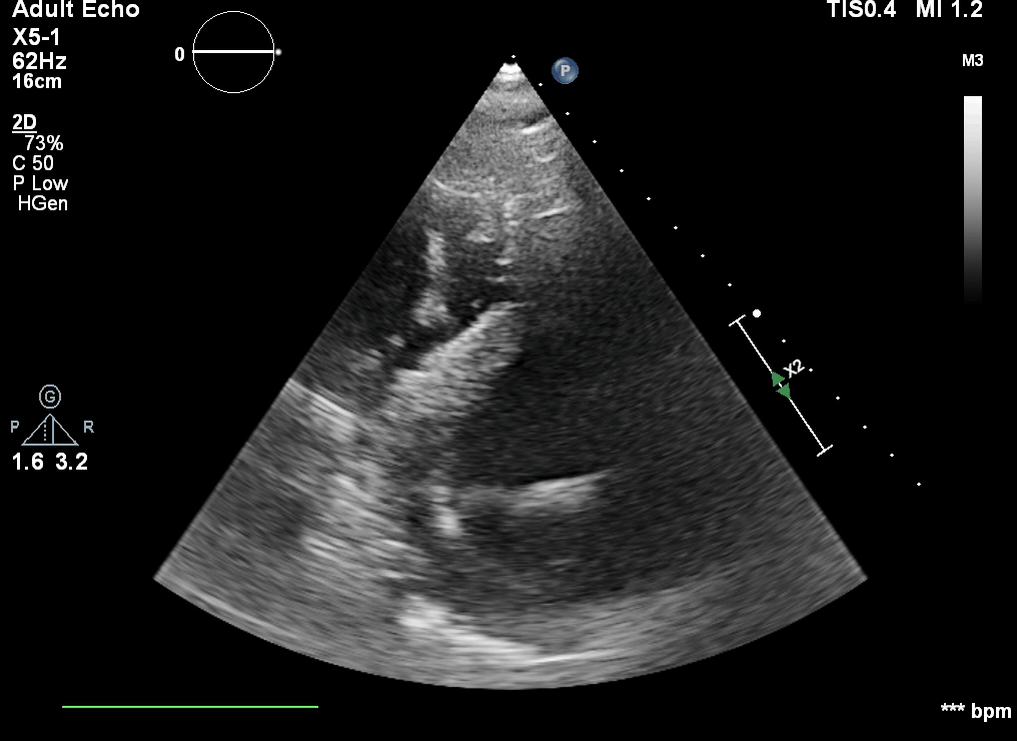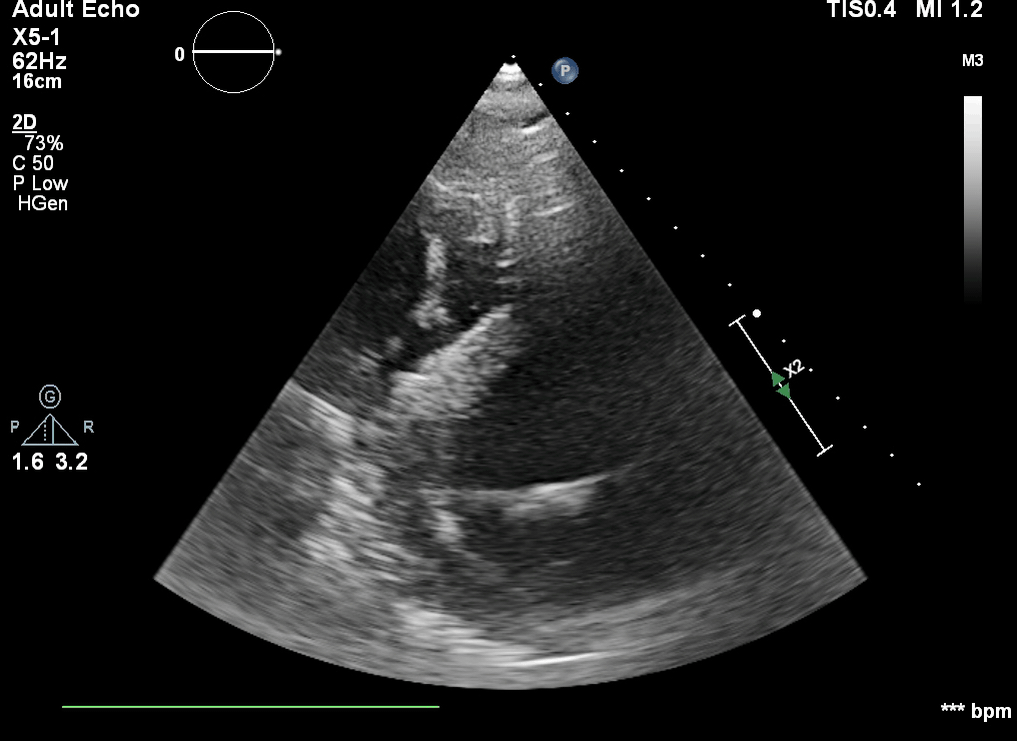
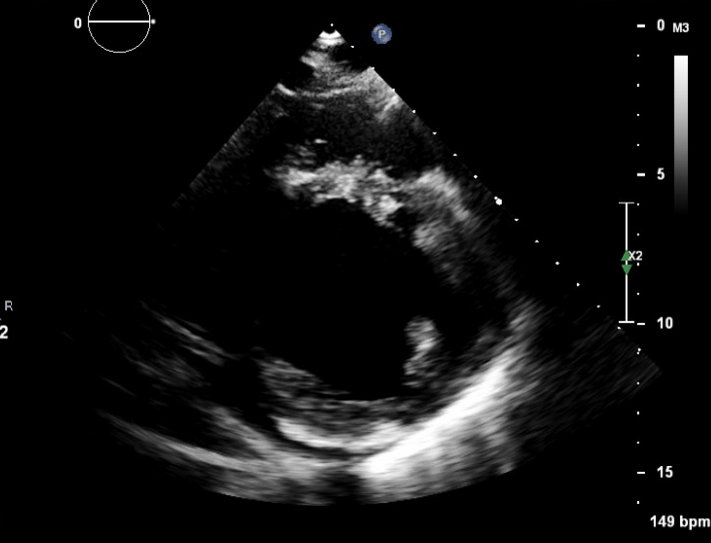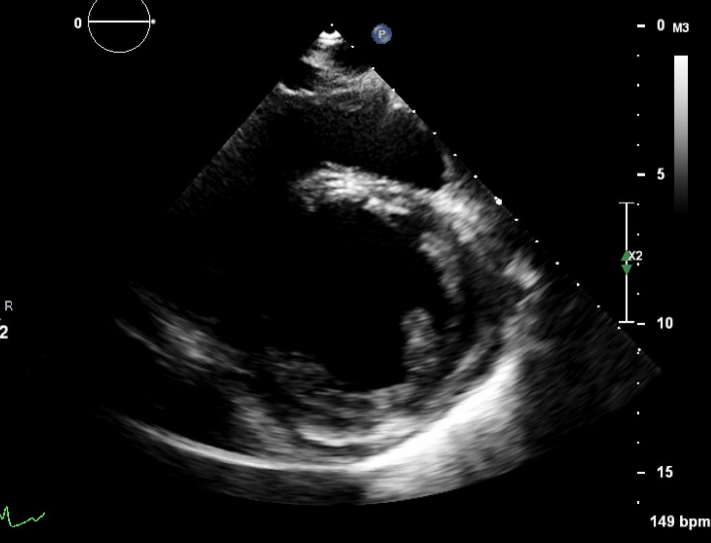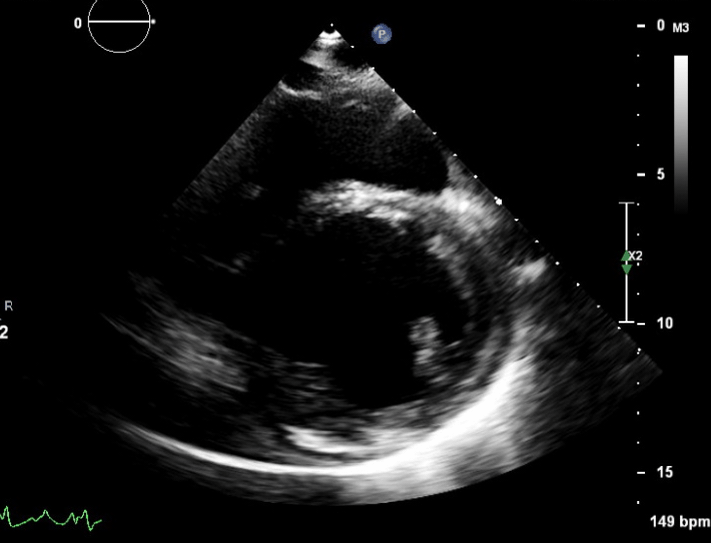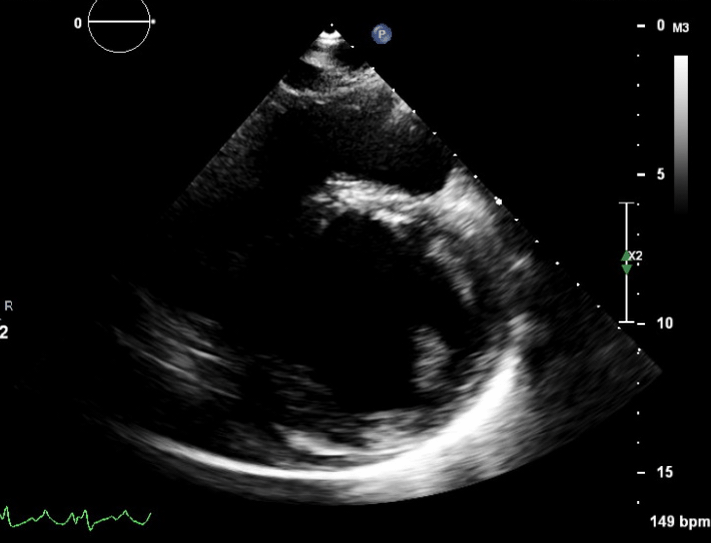
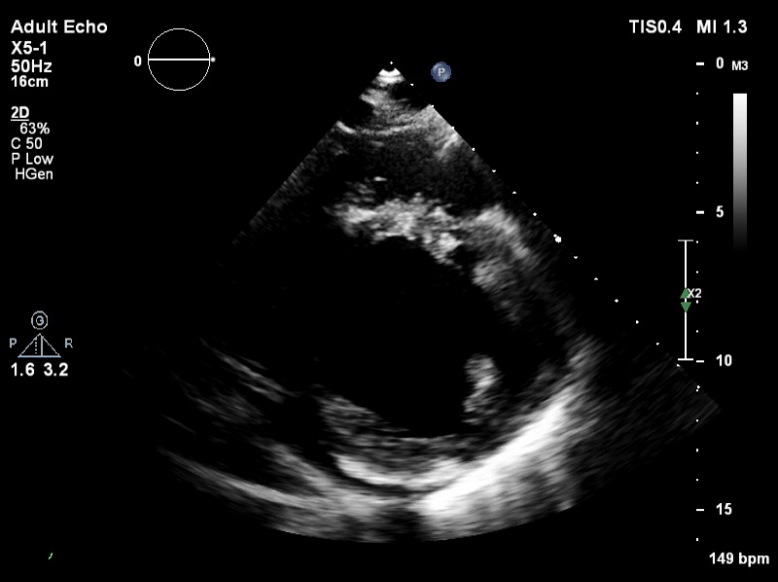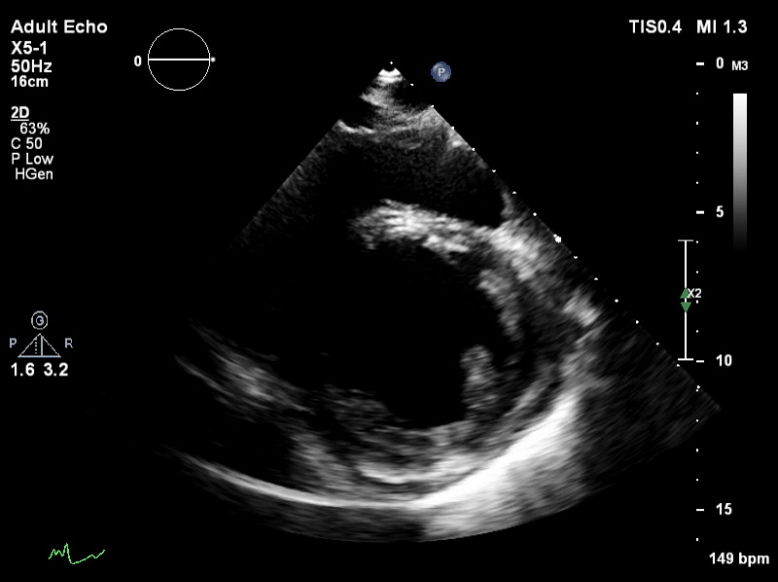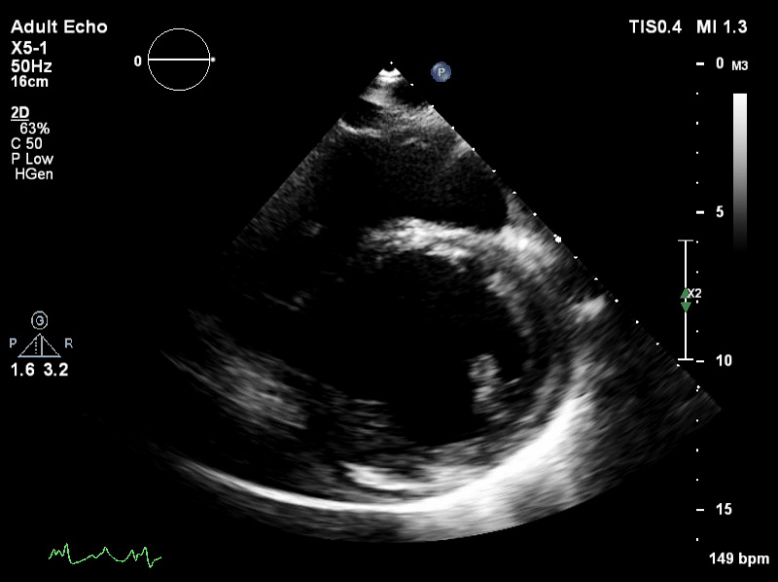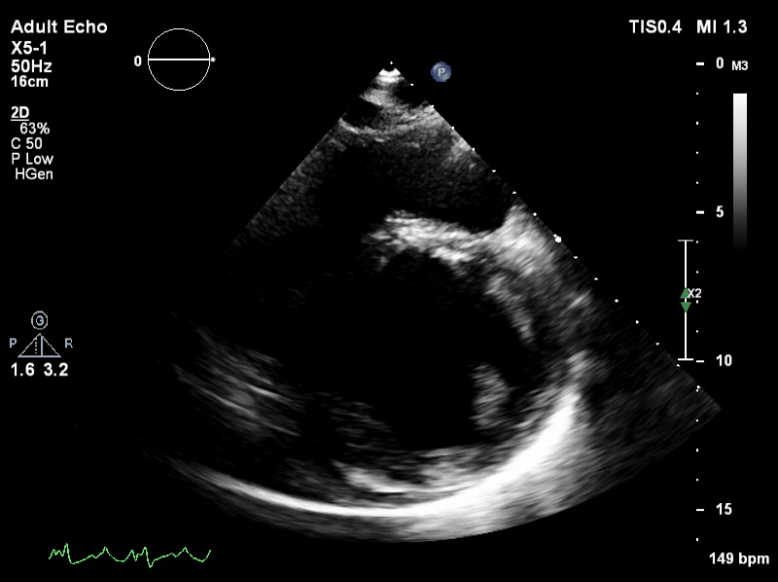
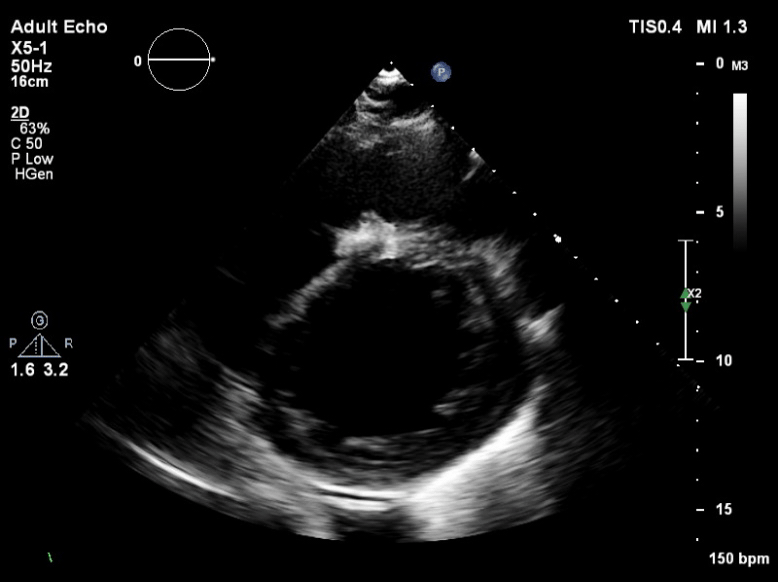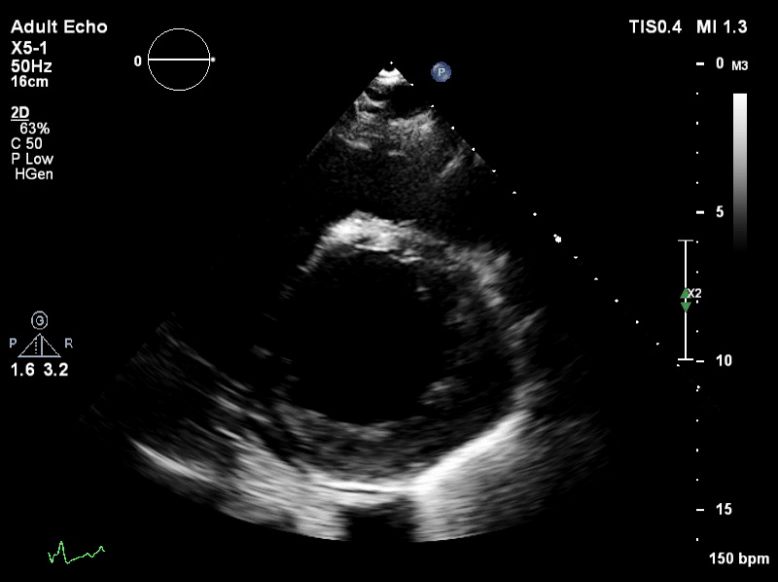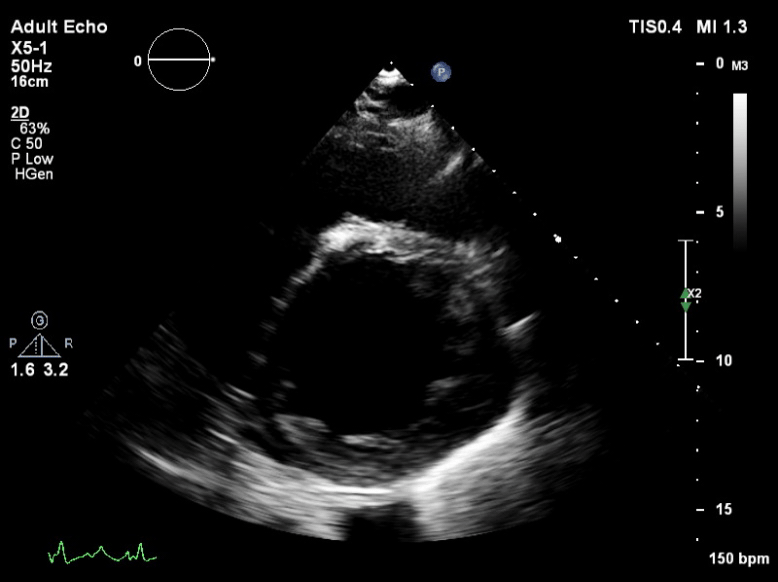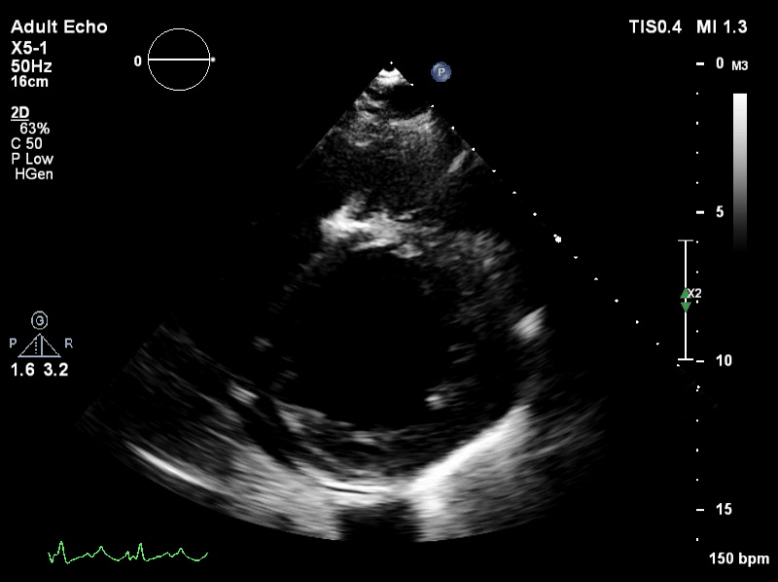
Q6. The following is seen on TTE. What are the implications of these echocardiographic findings?
These are TTE loops demonstrating severely reduced global left ventricular (LV) function.
There is inadequate LV unloading as evidenced by the following:
- distended and non-contractile LV
- aortic valve opening only intermittently or not at all
- bowing of the inter-atrial septum towards the right
- severe mitral regurgitation – may or may not be present, depending on degree of LV distention
The aortic valve (AV) should open at least once every few beats – failure to do so implies high risk of inadequate unloading. This may cause subsequent LV distention and/or LV thrombus formation, or even formation of thrombus around the AV5. TTE is useful in evaluating early thrombus formation in the LV, specifically when patients have an ejection fraction (EF) < 20%.
If the AV is only opening every second or third beat, the need for LV distension strategy would need to be assessed in the context of the overall risk – i.e. LV size, presence of pulmonary edema, pulmonary capillary wedge pressure (PCWP), absolute EF and left ventricular outflow tract velocity time integral (LVOT VTI).
See Everything ECMO 020 for more on this topic.
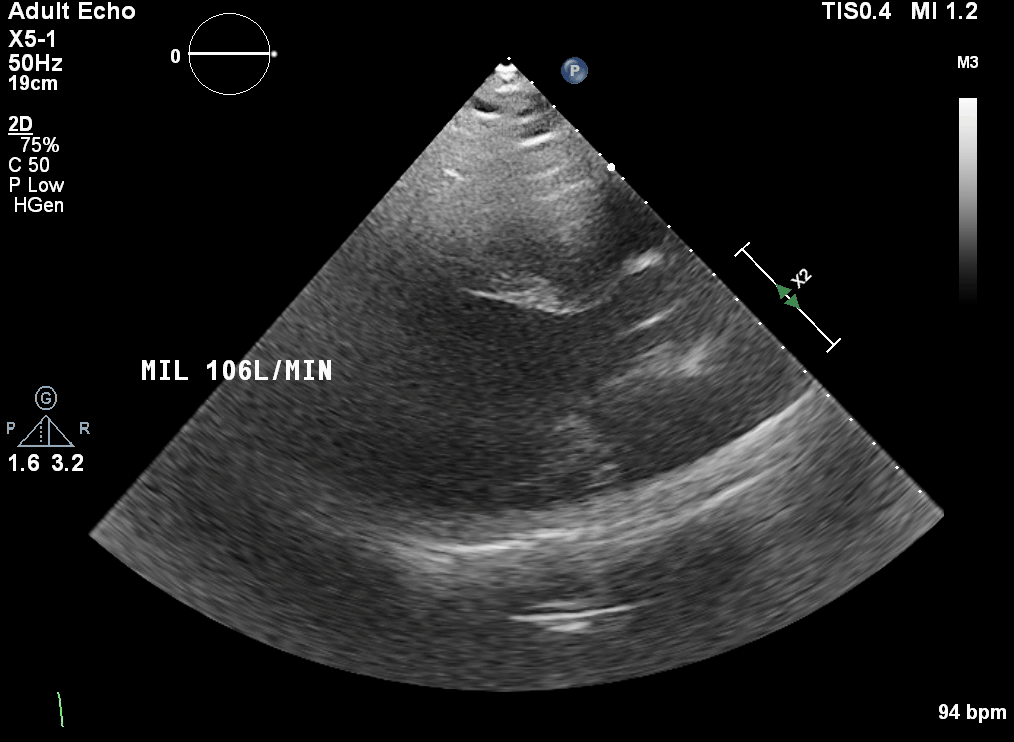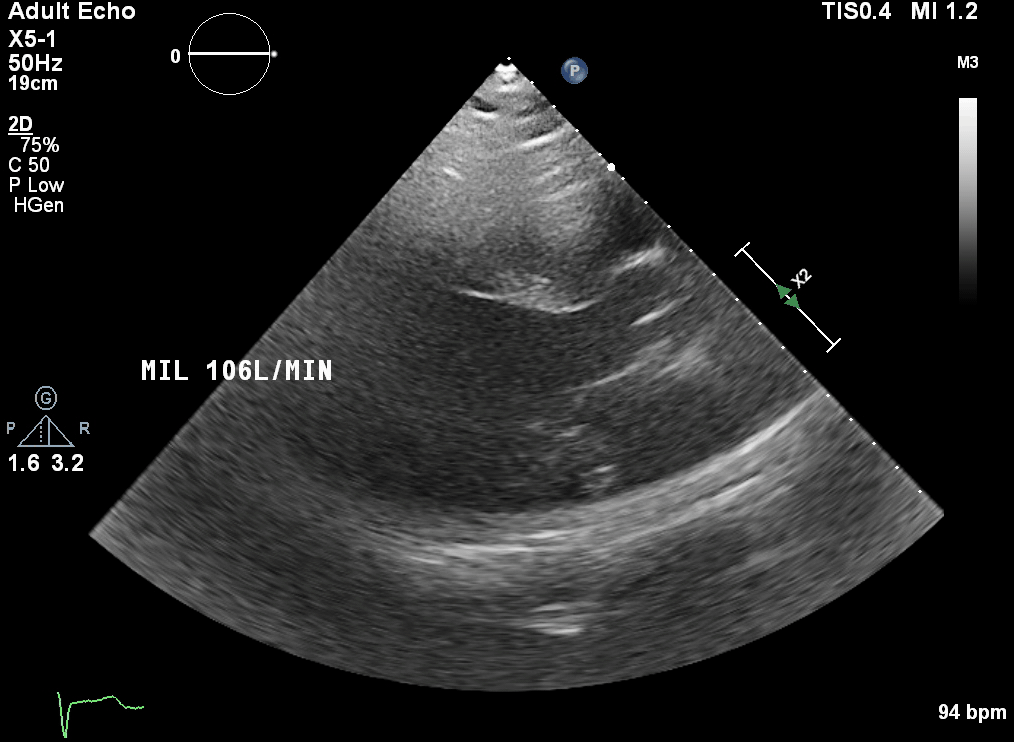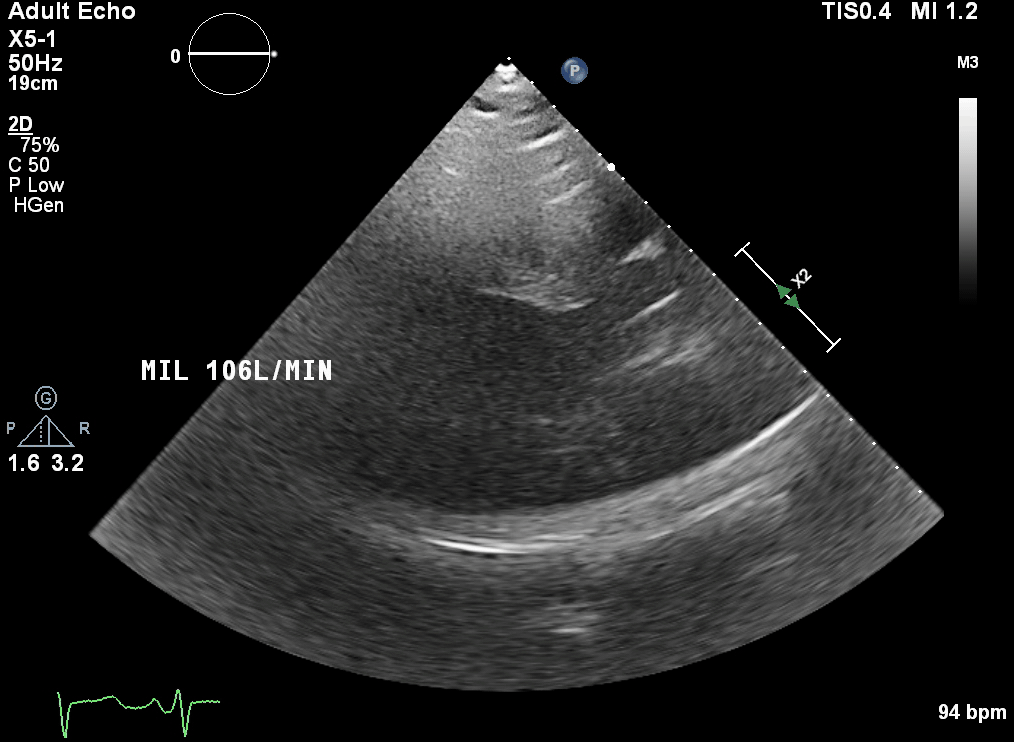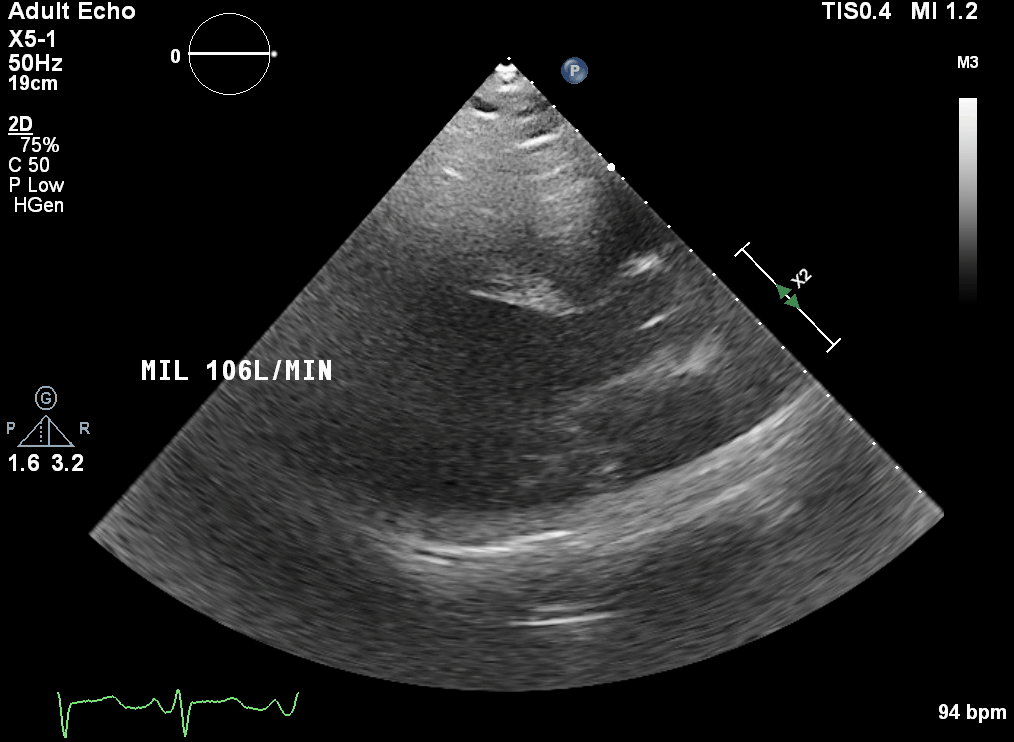
Q7. The ECHO below is recorded after inotropic support is increased. What does this show and what are the implications?
This is a TTE parasternal short axis (PSAX) view. It demonstrates a regional wall motion abnormality in the left anterior descending (LAD) territory, consistent with the patient’s initial infarct.
The diagram below demonstrates the coronary artery territories in the short axis views – from mid-papillary to basal6.
Grading of wall motion abnormalities using the segment system should be evaluated in multiple views and a four-grade scoring should be applied6:
- normal, or hyperkinetic
- hypokinetic (reduced thickening)
- akinetic (absent or negligible thickening)
- dyskinetic (systolic thinning or stretching/aneurysmal)
Left bundle branch block and paced rhythms make assessment of RWMAs difficult and must be taken into account when reporting the findings. The echocardiographic “pivot point” – where there is a change in the grade of wall motion, can be used to identify the location, and classify the location of wall motion abnormalities.
The implication of a dyskinetic LAD distribution wall motion abnormality in this patient who has had reperfusion, is that the affected myocardium will be unlikely to recover, and the patient will be unable to be weaned from mechanical support.
References
- Murphy D, Nanjayya VB. Ultrasound Guidance for Extra-Corporeal Membrane Oxygenation General Guidelines. ELSO Guideline (2015).
- Broch O, Renner J, Gruenewald M, et al. Variation of left ventricular outflow tract velocity and global end-diastolic volume index reliably predict fluid responsiveness in cardiac surgery patients. J Crit Care. 2012;27(3):325.e7-13. [pubmed]
- Kapoor PM. Echocardiography in extracorporeal membrane oxygenation. Ann Card Anaesth. 2017;20(Supplement):S1-S3. [pubmed] [article]
- Douflé G, Roscoe A, Billia F, Fan E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care. 2015;19:326. [pubmed] [article]
- Hireche-chikaoui H, Grübler MR, Bloch A, Windecker S, Bloechlinger S, Hunziker L. Nonejecting Hearts on Femoral Veno-Arterial Extracorporeal Membrane Oxygenation: Aortic Root Blood Stasis and Thrombus Formation-A Case Series and Review of the Literature. Crit Care Med. 2018;46(5):e459-e464. [pubmed]
- Lang RM, Badano LP, Mor-avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. [pubmed]