Everything ECMO 045: The role of ECPella in unloading the Left Ventricle
Author: Dr Sneha Varkey
Peer reviewer: Dr Vinodh Nanjayya
A 44-year-old female patient suffered an out-of-hospital ventricular fibrillation (VF) arrest. The time from her arrest to return of spontaneous circulation was 20 minutes. Coronary angiography revealed multiple thrombotic lesions in the LAD/D1/D2. Despite thromboaspiration, she had recurrent VF arrests with worsening cardiogenic shock (CS), so an Impella CP (Abiomed, Danvers, MA) was inserted through the right femoral artery for left ventricular (LV) support. Despite the Impella CP support, her cardiogenic shock (CS) progressed, and she was placed onto peripheral VA-ECMO with the Impella CP remaining in situ at setting P2*.
*P settings refer to different pump support settings on the Impella – to learn more about Impella basics, read Everything ECMO 034 and 035.
Q1. What is ECPella?
ECPella is the simultaneous use of VA-ECMO with Impella for the management of acute CS.
- It is mainly used in acute myocardial infarction-related CS patients at high risk of LV distension
- Generally, whilst on ECPella support, the Impella generates 1.5-2 L/min of flow to unload the LV (usually P2-P4 level).
Q2. Why would an Impella be inserted for a patient already established on VA-ECMO?
Reasons include:
- To actively unload the LV and prevent LV distension syndrome
- It may be considered in cases of pulmonary oedema, arrhythmias and evidence of blood stagnation within the LV
Q3. Why would a patient with an Impella in situ escalate to ECMO?
Reasons include:
- Progressive CS requiring increasing cardiac support above the capability of the inserted Impella. For example, an Impella 2.5 catheter’s maximum flow rate is 2.5 L/min which may be inadequate in a patient with evolving CS after protected PCI.
- Concomitant right heart failure1,2.
- Cardiac arrest
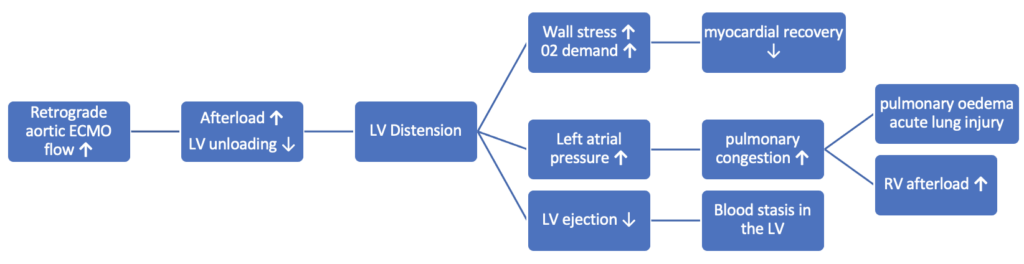
Q4. What is LV distension syndrome?
LV distension syndrome is a pathological increase in LV end-diastolic pressure (LVEDP) or volume (LVEDV) caused or exacerbated by VA ECMO.
- While ECMO can be life-saving in cardiogenic shock, the pathological increase in LVEDP and LVEDV that can sometimes affect patients on VA-ECMO can be disastrous and fatal if not addressed quickly3.
- The increased LVEDP can worsen subendocardial ischemia, which can jeopardise cardiac recovery.
- LV dilatation and pressure overload leads to increased left atrial pressure and pulmonary oedema4. Furthermore, if the LV dysfunction is severe the aortic valve (AV) may remain closed, even during systole, which can lead to stasis of blood in the LV and increasing the risk of intracardiac thrombus forming.
There are multiple contributing mechanisms:5,6
- During VA-ECMO the arterial return cannula generates retrograde flow in the aorta towards the aortic valve, resulting in increased afterload on the left ventricle (more than seen in a normal physiological state). This marked increase in afterload leads to increased LV wall stress, LV distension and increased myocardial 02 demand.
- The ECMO pump and retrograde flow towards the AV can worsen any aortic regurgitation, particularly if there is little or no LV ejection.
- Native cardiac output may be so poor that it is insufficient to eject the volume of blood entering the LV from the right side via the pulmonary veins.
Q5. How does ECPella work?
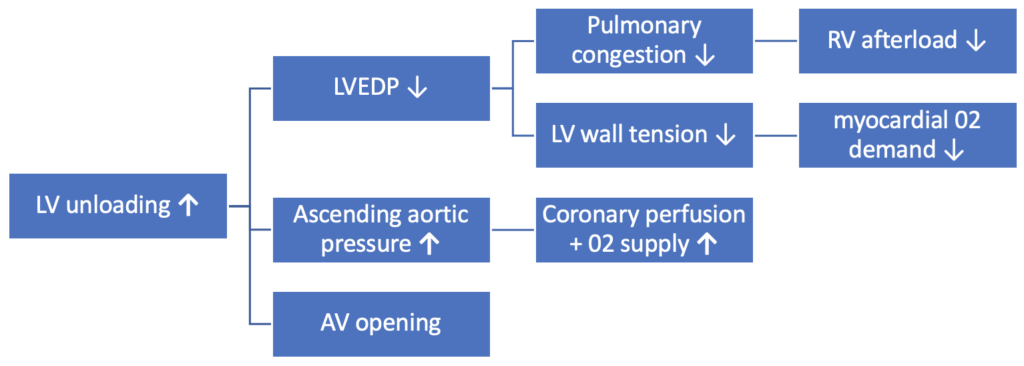
Impella has several important haemodynamic effects: increasing cardiac power output, improving 02 supply and decreasing 02 demand.
- The Impella outflow port sits in the aortic root and provides an active forward flow that helps to drain and unload the LV. The degree of forward flow is dependent on the pump support setting (P level) and the pressure gradient between the aorta and LV7.
- Coronary artery blood flow is influenced by the aortic pressure. In addition to increasing ascending aortic pressure, by draining the LV of blood the Impella reduces LVEDP and LVEDV – leading to reduced LV wall tension and less mechanical work. As a result myocardial 02 demand is reduced, whilst coronary perfusion and oxygen supply are increased8.
- As a result, the total balance of oxygen demand/delivery becomes more favourable9
Q6. Who is particularly at risk of LV distension?
Patients with:
- Predominantly left heart failure
- Advanced chronic cardiomyopathy
- Pre-existing aortic regurgitation
- Prolonged cardiac arrest
Q7. What other methods are there to prevent LV distension?
Measures used to prevent LV distention include:
- Medical management to reduce LV afterload
- Vasodilator therapy to reduce MAP to 60-70mmHg
- PEEP (>15cmH20)
- Medical management to support native contractility and maintain pulsatility
- Inotropes
- Manage circulating volume with fluid removal (diuresis, CVVH)
- Mechanical unloading therapies5
- Impella
- Trans-aortic LV catheter10
- Trans-apical surgical LV vent10
- Intra-aortic balloon pump
- Atrial septostomy (balloon or surgical)
See Everything ECMO 020 to learn more about LV distention.
Q8. What are the advantages of ECPella?
Advantages include:
- Impella insertion is percutaneous and can be performed before, during, or after VA-ECMO cannulation.
- Insertion can be performed in the operating theatre, cardiac lab, or bedside in ICU – particularly attractive for a patient who is critically unstable on VA-ECMO
- The additional flow provided by the Impella may facilitate ECMO weaning. As a result, ECMO may potentially be weaned faster – shortening the time on ECMO and the development of ECMO related complications11.
- There is a growing body of evidence suggesting that patients supported with this strategy have not only lower 30-day mortality and lower inotrope use, but also demonstrate a trend towards higher LV ejection fraction after weaning of support when compared with VA-ECMO alone12, 13. However, there is currently no data available from randomised controlled trials to confirm these findings.
Q9. What are the disadvantages of ECPella?
Disadvantages include:
- Cost and availability
- Increased haemolysis and bleeding when compared to VA-ECMO alone11
- Increased incidence of insertion site-related complications – both minor bleeding and limb ischemia14
- Migration and malposition can frequently occur, requiring regular echocardiographic studies to reposition
- In patients with Impella on ECMO, the delivery of blood flow to the LV from the right ventricle (RV) is reduced as most of the venous return is diverted to the ECMO from the right atrium (RA). This may lead to LV suction even when the level is low at P2. Therefore, VA ECMO support may need to be reduced to allow more blood flow delivery to the LV. If feasible, VA-ECMO flow of 3-4 L/min (partial support) should be maintained to preserve native cardiac contractility and avoid suction events9. Adequate anticoagulation therefore needs to be ensured to prevent any thrombus formation in either the Impella catheter or the VA ECMO circuit as both devices are likely to be on partial support.
References
- Remmelink M, Sjauw KD, Henriques JP, de Winter RJ, Koch KT, van der Schaaf RJ, et al. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70(4):532-7. PMID: 17896398
- Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, et al. Concomitant implantation of Impella. Eur J Heart Fail. 2017;19(3):404-12. PMID: 27709750
- Cevasco M, Takayama H, Ando M, Garan AR, Naka Y, Takeda K. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J Thorac Dis. 2019;11(4):1676-83. PMID: 31179113
- Lüsebrink E, Orban M, Kupka D, Scherer C, Hagl C, Zimmer S, et al. Prevention and treatment of pulmonary congestion in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Eur Heart J. 2020;41(38):3753-61. PMID: 33099278
- Soleimani B, Pae WE. Management of left ventricular distension during peripheral extracorporeal membrane oxygenation for cardiogenic shock. Perfusion. 2012;27(4):326-31. PMID: 22473862
- Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, et al. Incidence and Implications of Left Ventricular Distention During Venoarterial Extracorporeal Membrane Oxygenation Support. ASAIO J. 2017;63(3):257-65. PMID: 28422817
- Lim HS. The Effect of Impella CP on Cardiopulmonary Physiology During Venoarterial Extracorporeal Membrane Oxygenation Support. Artif Organs. 2017;41(12):1109-12. PMID: 28591467
- Belohlavek J, Hunziker P, Donker DW. Left ventricular unloading and the role of ECpella. Eur Heart J Suppl. 2021;23(Suppl A):A27-A34. PMID: 33815012
- Meani P, Lorusso R, Pappalardo F. ECPella: Concept, Physiology and Clinical Applications. J Cardiothorac Vasc Anesth. 2022;36(2):557-66. PMID: 33642170
- The Alfred Hospital M. Alfred ECMO Guideline [Available from: https://ecmo.icu/va-ecmo-lv-distension-syndrome/.
- Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation. 2020;142(22):2095-106. PMID: 33032450
- Patel SM, Lipinski J, Al-Kindi SG, Patel T, Saric P, Li J, et al. Simultaneous Venoarterial Extracorporeal Membrane Oxygenation and Percutaneous Left Ventricular Decompression Therapy with Impella Is Associated with Improved Outcomes in Refractory Cardiogenic Shock. ASAIO J. 2019;65(1):21-8. PMID: 29489461
- Shibasaki I, Masawa T, Abe S, Ogawa H, Takei Y, Tezuka M, et al. Benefit of veno-arterial extracorporeal membrane oxygenation combined with Impella (ECpella) therapy in acute coronary syndrome with cardiogenic shock. J Cardiol. 2022;80(2):116-24. PMID: 35288000
- Nakamura M, Imamura T. Practical Management of ECPELLA. Int Heart J. 2020;61(6):1094-6.
- Amarelli C, Musumeci F, Loforte A, Andrea Montalto A, Franco SD, Hernandez-Montfort J. Flow Optimization, Management, and Prevention of LV Distention during VA-ECMO. 2016. In: Advances in Extra-corporeal Perfusion Therapies [Internet]. Available from: https://www.intechopen.com/chapters/64682 doi: 10.5772/intechopen.80265.
All case-based scenarios on INTENSIVE are fictional. They may include realistic non-identifiable clinical data and are derived from learning points taken from clinical practice. Clinical details are not those of any particular person; they are created to add educational value to the scenarios.