Author: Dr Sean Keane
Peer reviewers: Dr Vinodh Nanjayya, A/Prof Chris Nickson
Everything ECMO 034
A 58-year-old female with an acute myocardial infarction (AMI) has a successful percutaneous cardiac intervention (PCI) to a proximal left anterior descending (LAD) lesion in the cath lab. Post procedure she is in cardiogenic shock. The bedside echo shows a severely impaired left ventricle (LV) with preserved right ventricular (RV) contractility and no major valvular pathology. The hospital is out of extracorporeal membrane oxygenation (ECMO consoles) due to the COVID-19 crisis, and the conversation shifts to inserting an Impella®.
Part 1 discusses principles, indications, insertion and positioning of an Impella®. Part 2 will will discuss contraindications, anticoagulation management, complications and evidence.
Q1. What are the indications for Impella® insertion?ECMO?
An Impella® is a short-term percutaneous ventricular assist device (pVAD) that is indicated for:
- Haemodynamic support during high-risk percutaneous coronary intervention (PCI), otherwise termed “protected PCI”
- Cardiogenic shock due to AMI, severe heart failure, or cardiac surgery
- Impella RP® is indicated for acute right heart failure following left ventricular assist device (LVAD) insertion, AMI, heart transplant, or cardiac surgery
Q2. Is an Impella® one size fits all?
No.
Numerous Impella® devices exist, which can be broadly thought of as left or right-sided devices. Devices can be inserted percutaneously via the femoral artery or femoral vein, or surgically via the femoral artery, axillary artery or ascending aorta.
The choice of Impella® depends on the clinical situation and whether the patient requires left ventricular, right ventricular, or bi-ventricular support.
| Impella® Model | Circulatory Support | Inlet | Outlet | Peak Flow (L/min) | Max Days |
| 2.5 | Left | LV | Ascending aorta | 2.5 | 4 |
| CP | Left | LV | Ascending aorta | 4.3 | 4 |
| 5.0 | Left | LV | Ascending aorta | 5.0 | 14 |
| 5.0 LD | Left | LV | Ascending aorta | 5.0 | 14 |
| 5.5 | Left | LV | Ascending aorta | 6.2 | 14 |
| RP | Right | Inferior vena cava | Pulmonary artery | >4.0 | 14 |
*CP = Cardiac Power; LD = Left Direct; RP = Right Percutaneous
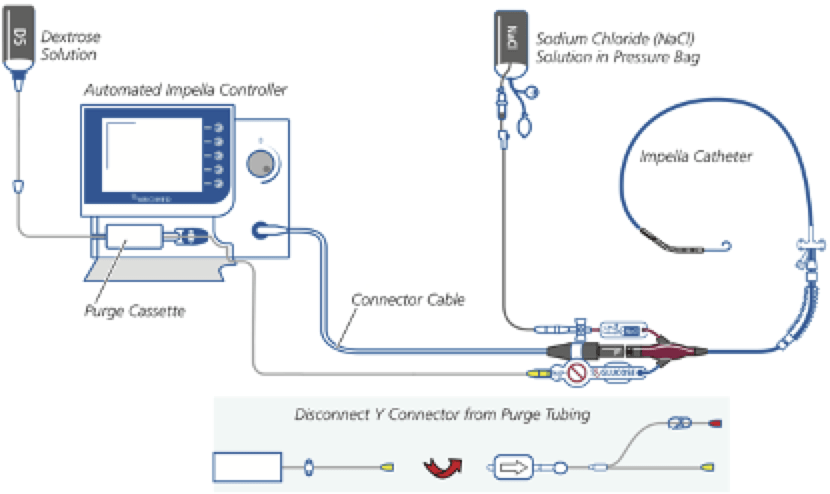
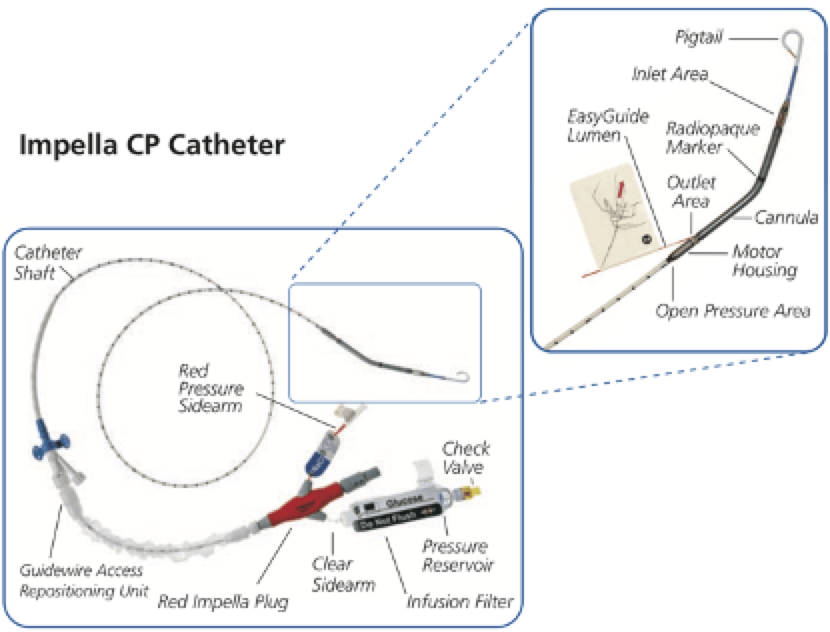
Q3. What are the main components of an Impella® system?
Q4. How does an Impella® work?
The Impella® is a catheter mounted micro-axial pump that uses the Archimedes’ screw principle to pump blood between the inlet and outlet.
Impella® devices designed to support the systemic circulation, namely the 2.5®, CP®, 5.0®, 5.0 LD® and 5.5® versions, draw blood from the LV and pump it into the ascending aorta. The LV is unloaded with increased cardiac output. This reduces myocardial oxygen consumption, increases mean arterial pressure and end organ perfusion, and reduces pulmonary capillary wedge pressure (PCWP) and the risk of acute pulmonary oedema. Adequate RV function is needed to maintain LV preload and haemodynamic integrity, and any dysfunction may become more apparent once the Impella® is inserted and the LV is unloaded. In a similar vein, ventricular tachycardia or fibrillation will render the Impella® ineffective due to RV failure.
The Impella RP® is designed to support the pulmonary circulation, and pumps blood from the inferior vena cava (IVC) into the pulmonary artery.
AIC provides visual information, enables manipulation of P levels (RPM) to change blood flow, and controls the purge system.
Q5. Should patients be awake at the end of their life on ECMO?
This patient is in cardiogenic shock due to AMI despite successful revascularisation of the LAD territory. She requires systemic circulatory support, but is maintaining some native cardiac output. An Impella CP® inserted percutaneously via the femoral artery in the cath lab is the preferred choice.
Q6. How is an Impella® inserted and positioned?
An Impella® is typically inserted in the cath lab, ICU or operating theatre. The clinical situation and location of insertion will determine device choice. Position is confirmed with fluoroscopy (cath lab), echocardiography (ICU/theatre), and haemodynamic waveform data on the AIC (all).
| Impella® Model | Insertion Location (Typical) | Method of Insertion | Inlet Position | Outlet Position |
| 2.5 CP* | Cath lab (*or ICU) | Percutaneously via femoral artery sheath | 3.5cm below the aortic valve annulus in the mid-ventricular cavity | Ascending aorta |
| 5.0 | Cath lab, ICU or theatre | Percutaneous cut-down via femoral artery | 3.5cm below the aortic valve annulus in the mid-ventricular cavity | Ascending aorta |
| 5.0 LD 5.5 | Theatre | Surgical insertion via axillary artery or ascending aorta using tunneled side-to-side Dacron tube graft | 3.5cm below the aortic valve annulus in the mid-ventricular cavity | Ascending aorta |
| RP | Cath lab, ICU or theatre | Percutaneously via femoral vein sheath | Outside right atrium at or below level of the diaphragm | 2-4cm above the pulmonic valve |
Devices with SmartAssist® technology have integrated haemodynamic sensors in the catheter and allow re-positioning in the ICU without echocardiography in the event of device malposition.
Devices with SmartAssist® technology have integrated haemodynamic sensors in the catheter and allow re-positioning in the ICU without echocardiography in the event of device malposition.
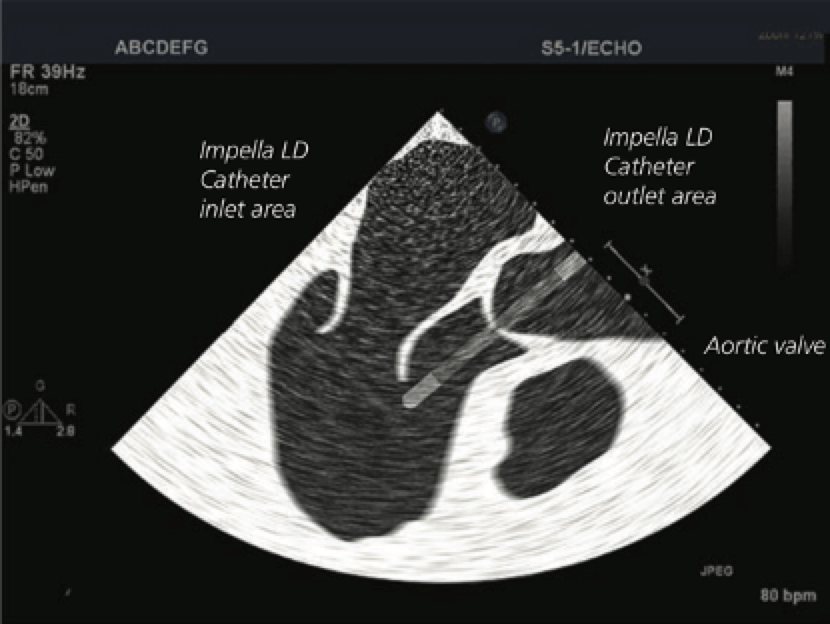
Figure 3. Transoesophageal echocardiogram illustration showing correct Impella LD® catheter position
The following video provides additional information for using imaging and the AIC to manage Impella® positioning:
Continue on to Part 2.
References
- ABIOMED [Internet]. 2020 Aug 25; Available from: https://www.abiomed.com
- Amin AP, Spertus JA, Curtis JP, Desai N, Masoudi FA, Bach RG, McNeely C, Al-Badarin F, House JA, Kulkarni H, Rao SV. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020 Jan 28;141(4):273-84.
- Griffith BP, Anderson MB, Samuels LE, Pae Jr WE, Naka Y, Frazier OH. The RECOVER I: a multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. The Journal of thoracic and cardiovascular surgery. 2013 Feb 1;145(2):548-54.
- Khalid N, Rogers T, Shlofmitz E, Chen Y, Musallam A, Khan JM, Iantorno M, Gajanana D, Hashim H, Torguson R, Bernardo N. Adverse events and modes of failure related to Impella RP: insights from the Manufacturer and User Facility Device Experience (MAUDE) Database. Cardiovascular Revascularization Medicine. 2019 Jun 1;20(6):503-6.
- Lauten A, Engström AE, Jung C, Empen K, Erne P, Cook S, Windecker S, Bergmann MW, Klingenberg R, Lüscher TF, Haude M. Percutaneous left-ventricular support with the Impella-2.5–assist device in acute cardiogenic shock: results of the Impella–EUROSHOCK-registry. Circulation: Heart Failure. 2013 Jan;6(1):23-30.
- Mario I, Albani S, Giannini F, Colangelo S, Boccuzzi GG, Roberto G, Brilakis ES, de Ferrari GM, Colombo A. Short term outcomes of Impella in cardiogenic shock: A review and meta-analysis of observational studies. International Journal of Cardiology. 2020 Sep 22.
- O’Neill WW, Grines C, Schreiber T, Moses J, Maini B, Dixon SR, Ohman EM. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. American heart journal. 2018 Aug 1;202:33-8.
- Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. Journal of the American College of Cardiology. 2017 Jan 16;69(3):278-87.
- Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. Journal of the American College of Cardiology. 2015 May 19;65(19):e7-26.
- Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. Journal of the American College of Cardiology. 2008 Nov 4;52(19):1584-8.
- Shariff M, Doshi R, Pedreira Vaz I, Adalja D, Krishnan A, Hegde S, Kumar A. Impella versus intra-aortic balloon pump in cardiogenic shock: a meta-analysis assessing 30-days mortality. European Heart Journal. 2020 Nov;41(Supplement_2):ehaa946-1130.
All case-based scenarios on INTENSIVE are fictional. They may include realistic non-identifiable clinical data and are derived from learning points taken from clinical practice. Clinical details are not those of any particular person; they are created to add educational value to the scenarios.