Everything ECMO 039: V-V ECMO Indications, Triggers and Patient Selection
Author: Dr Charlene Chua
Reviewer: A/Prof Chris Nickson
A 50-year-old man presented to a regional hospital 3 days ago with fever, shortness of breath and cough. He was subsequently diagnosed with community-acquired pneumonia. He had progressive respiratory failure necessitating intubation, mechanical ventilation, deep sedation and neuromuscular blockade. His chest X-ray showed extensive bilateral infiltrates. He remained profoundly hypoxaemic (P/F ratio 75) despite optimisation of ventilator strategies, antibiotics treatment and appropriate fluid balance management. He required low-dose vasopressor support. The hospital made a referral for V-V ECMO initiation.
Q1: What is the physiological rationale for V-V ECMO in respiratory failure?
The physiological rationale for V-V ECMO is based on its ability to:
- Improve systemic oxygenation
- Increase systemic carbon dioxide removal
- Facilitate lung rest
- Prevent injurious mechanical ventilation
V-V ECMO provides full or partial support for the patient while offering time for specific treatment to work and allowing native lung recovery from the underlying reversible condition to occur.1-3
Q2. What are the clinical indications for V-V ECMO initiation?
V-V ECMO should be considered for patients with acute, life-threatening, reversible respiratory failure that has not responded to conventional therapy.
Two common clinical indications for V-V ECMO are:
- Hypoxaemic respiratory failure with a ratio of partial arterial oxygen pressure to the fraction of inspired oxygen (PaO2/FiO2) of < 80 mmHg despite optimisation of medical management; and
- Hypercapnic respiratory failure with arterial pH < 7.25 despite conventional mechanical ventilation settings optimisation.
Less common indications for V-V ECMO include:
- Ventilatory support as a bridge to lung transplantation1
- Lung hyperinflation e.g. status asthmaticus4
- Severe air leak syndrome e.g. large bronchopleural fistula
- Trauma e.g. airway disruption or compression, chest injury with pulmonary contusion
- Intra-operative support for complex airway and lung surgery5
Q3. What factors influence the decision to commence V-V ECMO for this patient?
General principles:
- Ensure that the eligibility criteria for V-V ECMO are met.
- Ensure that there are no contraindications for V-V ECMO.
- All conventional therapies have been tried unsuccessfully with ongoing physiological deterioration.
- Reversible conditions such as pneumothorax, pleural effusion and mucous plugging have been treated or excluded.
- Cardiovascular function is optimised and preserved.
- It is consistent with the patient’s wishes.
- There is agreement from the involved specialty teams.
Specific factors for consideration:6
- Patient factors
- Age
- Pre-existing medical comorbidities (see contraindications below)
- Frailty/ baseline functional status
- Patient’s wishes/ advanced care directives
- Body habitus (practical challenges in cannulation)
- Suitable anatomy for percutaneous cannulation
- Disease factors
- Reversibility of underlying lung condition
- Complications of the disease
- Acuity and duration of disease
- Rate of lung injury progression
- Other organ failures
- Acute shock state
- Institutional factors
- Availability of resources/ staff
- Need for retrieval and inter-hospital transport
4. What are the principles guiding patient selection for V-V ECMO?
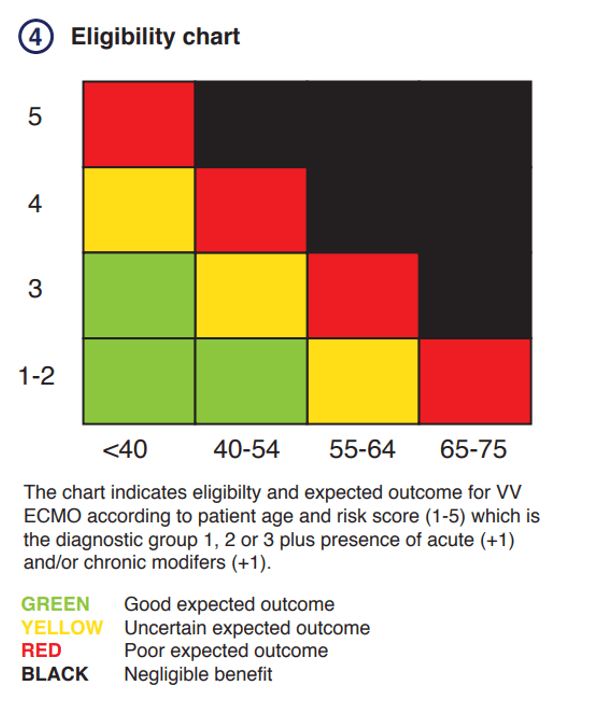
An eligibility chart can be used to establish suitability and expected patient outcomes for V-V ECMO based on the patient’s age and calculated risk score. The risk score will be determined by the diagnostic group and the presence or absence of acute and/or chronic disease modifiers.
Step 1: Determine the diagnostic group
a. Favourable diagnostic categories (Score = 1)
- Community-acquired pneumonia (infective cause)
- Aspiration pneumonitis
- Status asthmaticus
- Primary graft dysfunction following lung transplant within 7 days
- Adult respiratory distress syndrome [ARDS] from primary pulmonary causes (excluding trauma)
b. High-risk diagnostic categories (Score = 2)
- Necrotising pneumonia
- Pulmonary vasculitis (Goodpasture’s, ANCA-associated, other autoimmune disease)
- Lung transplant recipient 7-30 days post transplant
- Traumatic injuries
- Moderate TBI with hypoxia/chest injury to allow neurological assessment
- Bronchial tear with air leak and hypoxia
- ARDS from direct chest trauma
c. Unfavourable diagnostic categories (Score = 3)
- Invasive aspergillosis
- Pneumocystis jirovecii pneumonia
- ARDS from non-pulmonary cause (e.g. burns, pancreatitis)
- Lung transplant recipients >30 days and suitable for re-transplantation
Step 2: Assess the clinical modifiers
a. Acute clinical modifiers – one or more present (Score = 1)
- Lactate ≥ 10
- Noradrenaline > 1 mcg/kg/min
- AST or ALT > 1000 μmol/L
- INR > 3.0
- Anuria > 4 hours
b. Chronic disease modifiers – one or more present (Score = 1)
- Peripheral vascular disease (symptomatic, revascularisation or amputation)
- Previously known ischaemic heart disease or prior revascularisation
- Prior valve surgery, CABG or aortic surgery
- Moderate COPD (GOLD Stage II, FEV1 50 – 79% predicted)
- Chronic renal failure stage 3 or 4 CKD
- Chronic liver disease
- Long-term immunosuppression
Step 3: Ensure no absolute contraindications
a. Lung disease
- Severe chronic lung disease
- Acute/ subacute pulmonary fibrosis as likely cause of respiratory failure
- Previously known/ treated SLE, extra-articular rheumatoid arthritis, scleroderma, dermatomyositis, sarcoidosis
- Clinical course or pathological investigations suggestive of an irreversible process (e.g. bleomycin-induced lung injury)
- Obliterative bronchiolitis as likely cause of respiratory failure
- Graft versus host lung disease
b. Patient profile
- Age >75 years
- Patient’s specific wishes against the institution of extracorporeal support
- Bone marrow transplant recipients
- Terminal illness
- Disseminated malignancy with limited prospects of survival
- Liver cirrhosis Child-Pugh B or C or decompensation (jaundice/ ascites/ encephalopathy)
- Severe intra-cranial injury/ haemorrhage
- End-stage heart failure, cardiomyopathy (VAD/ inotropes)
- Chronic renal failure CKD 5 or dialysis
c. Acute condition
- Pulmonary oedema / left heart failure – consider VA ECMO
- Septic shock with hypoxia predominant presentation rather than primary pulmonary infiltrates
- Advanced microcirculatory failure with severe mottling or established purpura
- Established multiorgan failures
Step 4: Estimate the expected outcome based on the eligibility chart
Patients with good expected outcomes (green) are suitable for V-V ECMO if clinically indicated. Patients with uncertain or sometimes poor expected outcomes (yellow or red) may still be suitable for V-V ECMO on a case-by-case basis in consultation with ECMO clinical specialists taking careful consideration of the overall risk-benefit profile and the patients’ values. V-V ECMO should not be initiated for patients expected to receive negligible benefit from it (black).
5. What mortality prediction systems can be used to facilitate patient selection for V-V ECMO?
Multiple mortality prediction systems such as RESP7, PRESERVE8, PRESET9 and ECMOnet10 have been established using data from small cohort of patients on V-V ECMO to estimate patients’ likelihood of survival. Most predictive systems have moderate discrimination at best between survivors and non-survivors on V-V ECMO11. These survival models might be useful at the population level, however, have little clinical utility at the bedside. The predictive systems should not be used solely for patient selection. Instead, the models serve to support the critical decision-making process taking into consideration the complexity of patient factors, functional status and values, clinical conditions as well as other non-clinical aspects.
6. Would you initiate V-V ECMO for this patient?
Yes (we would!)
V-V ECMO was initiated by the ECMO retrieval team at the regional hospital, The patient was transferred for ongoing management in an ECMO centre.
References
- Tonna, JE, Abrams, D, Brodie, D et al. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (V-V ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO Journal. 2021: 600-10. Available from Management_of_Adult_Patients_Supported_with.1.pdf (elso.org)
- Gattinoni, L, Vassalli, F, Romitti, F et al. Extracorporeal gas exchange: when to start and how to end?. Crit Care 2019;23(203). doi.org/10.1186/s13054-019-2437-2
- Ficial B, Vasques F, Zhang J, et al. Physiological Basis of Extracorporeal Membrane Oxygenation and Extracorporeal Carbon Dioxide Removal in Respiratory Failure. Membranes (Basel). 2021;11(3):225. doi:10.3390/membranes11030225
- Makdisi, G & Wang, I. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166-76. doi:10.3978/j.issn.2072-1439.2015.07.17
- Kelly, B & Carton, E. Extended Indications for Extracorporeal Membrane Oxygenation in the Operating Room. Journal of Intensive Care Medicine. 2020;35(1):24-33. doi:10.1177/0885066619842537
- Alfred Health ECMO Guideline. V-V ECMO: Patient Selection. [Internet]. Victoria (AU): 2020 [updated 2020; cited 2022 April 12] Available from: Patient selection – Alfred ECMO Guideline
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189(11):1374-82. doi: 10.1164/rccm.201311-2023OC
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704-13. doi: 10.1007/s00134-013-3037-2
- Hilder M, Herbstreit F, Adamzik M, et al. Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: the PREdiction of Survival on ECMO Therapy-Score (PRESET-Score). Crit Care. 2017;21(1):301. doi: 10.1186/s13054-017-1888-6
- Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39(2):275-81. doi: 10.1007/s00134-012-2747-1
- Fisser C, Rincon-Gutierrez LA, Enger TB, Taccone FS, Broman LM, et al. Validation of Prognostic Scores in Extracorporeal Life Support: A Multi-Centric Retrospective Study. Membranes (Basel). 2021;11(2):84. doi: 10.3390/membranes11020084