Author: Arne Diehl
Reviewers: Chris Nickson, Aidan Burrell
Everything ECMO 007
“Can you help?”
A 41 year-old man is supported with peripheral V-A ECMO. He presented 3 days ago with a STEMI, the percutaneous coronary intervention was complicated by pulmonary aspiration requiring intubation. Despite revascularisation he had progressive cardiogenic shock and was placed emergently on V-A ECMO. The circuit is running without any problems but he is now hypoxaemic with an arterial O2 saturation of 82%.
Q1. How do you assess this patient at the bedside?
As always, assess both “the man and the machine” (the patient and the ECMO support).
Patient
- Is there pulsatility? The degree of pulsatility observed on the arterial trace reflects intrinsic cardiac function
- Where was the oxygen saturation measured from? The oxygen saturation is routinely measured on the right arm in femoral V-A ECMO (alternatively the right ear lobe or forehead). If abnormal, the opposite site should be checked for comparison. A pre-oxygenator SaO2 can be used to exclude high oxygen extraction.
- Is there pulmonary dysfunction? Are there clinical findings of pulmonary oedema (LV failure) or other lung disease?
- Are ventilation settings appropriate? In particular, check FiO2, PEEP, and safe lung ventilation parameters
ECMO
- Is ECMO blood flow adequate to support the patient? Is there any change in parameters over time? (circuit blood flow, pump speed (rpm))
- Is oxygen being delivered to the oxygenator? Check fresh gas flow (FGF), blender and oxygen tubing connections (this is discussed for VV ECMO in Everything ECMO 001)
Your findings:
This patient has a femoro-femoral peripheral V-A ECMO configuration with unchanged circuit blood flow of 3.8L/min over the last day. The oxygen saturation on the right arm has fallen to 82% and measures 99% on the left hand. 100% oxygen is connected at a fresh gas flow of 5 L/min. There is now good pulsatility on the arterial trace. Ventilator settings are appropriate. Pre-oxygenator SaO2 is 75%, excluding excess oxygen extraction as a cause of hypoxaemia.
Q2. What is the likely diagnosis?
Differential hypoxia (aka Harlequin syndrome)
Differential hypoxia in this situation is expected due to the recovery of native cardiac function in the setting of aspiration causing respiratory failure.
You review the patient’s most recent chest x-ray and find persistent bilateral infiltrates consistent with aspiration pneumonia.
Q3. What is the underlying pathophysiology?
Patients on V-A ECMO have retrograde blood flow in the aorta, as blood moves up the aorta toward the heart from the ECMO circuit. When there is intrinsic cardiac activity there is also anterograde blood flow entering the aorta from the heart. The transition point where opposing flows meet in the aorta will vary and depend on the relative magnitude of the ECMO blood flow and the patient’s cardiac output, but may be as low as the mid aorta. In the presence of pulmonary disease, the blood leaving the heart may be poorly oxygenated due to impaired gas exchange in the lungs. We monitor SpO2 on the right upper limb as an early indicator that the transition point is moving distally because the right subclavian artery arises from the brachiocephalic artery, which is a proximal branch of the aorta. The transition point can also be visualised by contrast CT.
The transition point is shown in the aorta in the image below, at the level of the right atrium:
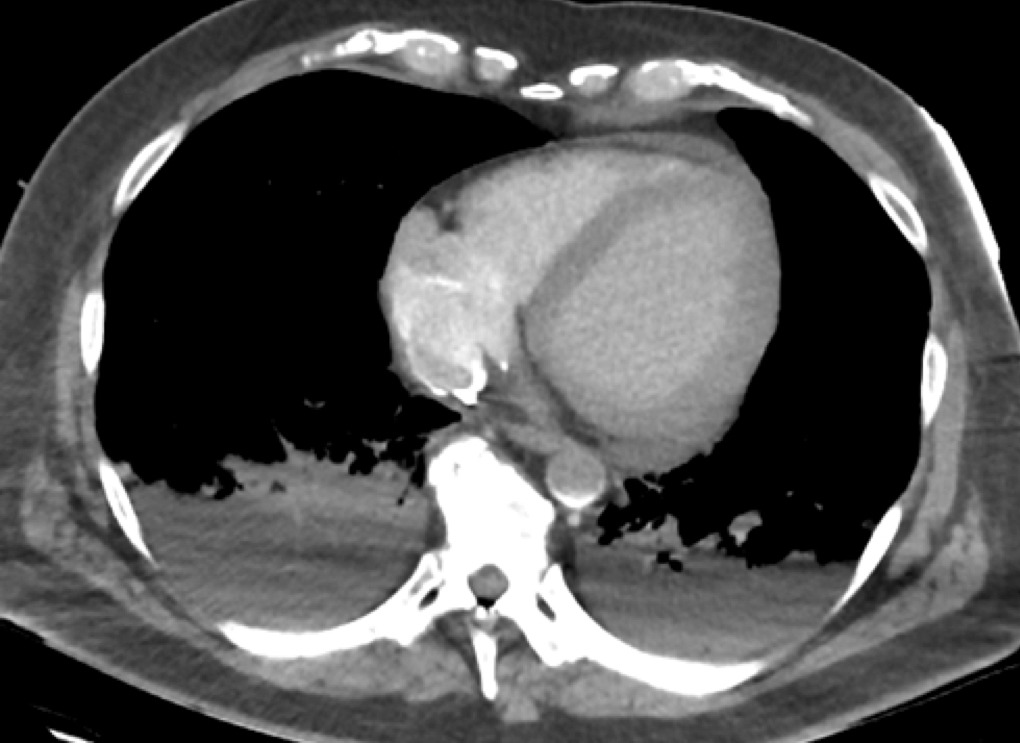
Transition point in V-A ECMO
Also note that:
- The bright rim of the access ECMO cannula is seen in the right atrium, most contrast is directly aspirated into the circuit
- The contrast rich blood returned via the femoral artery can be seen at the transition point in the aorta (sickle shape contrast in aorta) as a result of to retrograde flow from the ECMO return cannula.
Q4. What are the two major clinical concerns?
- Cardiac ischaemia
- Cerebral ischaemia
When there is differential hypoxaemia poorly oxygenated blood is ejected from the left ventricle and will first enter the coronary circulation with potential for cardiac ischaemia. If the transition point is more distal, poorly oxygenated blood will subsequently enter first the brachiocephalic and then the left carotid arteries, which may cause cerebral ischaemia.
Clinicians, paradoxically, should be alert to these potential complications whenever the heart begins to recover and pulsatility improves.
Q5. What are the prerequisites for this condition to occur and how do you confirm it?
Prerequisites for differential hypoxia to occur include:
- Patient on peripheral V-A ECMO
- Significant intrinsic cardiac output (indicated by good pulsatility present on arterial trace)
- Coexistant pulmonary dysfunction resulting in impaired oxygenation of blood flowing through the pulmonary circulation (indicated by imaging of the lungs, such as a chest x-ray, showing relevant pathology)
Differential hypoxia is suspected and diagnosed on clinical grounds in the presence of:
- The above prerequisites
AND
- Markedly lower oxygen saturation (SpO2) between the right arm compared to the left arm or between upper and lower limbs (the so-called “North-South phenomenon”). If in doubt this may need ABGs to confirm.
Q6. Can this condition occur in central ECMO?
No, patients on central V-A ECMO cannot develop differential hypoxia.
In central V-A ECMO the return cannula is sutured into the proximal aorta, with predominately anterograde blood flow down the aorta. There may be a drop in global oxygen saturation with increasing amount of deoxygenated blood ejected from the heart, however, significant differences in oxygen saturation between upper and lower body will not occur.
Q7. What is meant by the term ‘dual circulation’ in the context of this condition?
The concept of ‘dual circulation’ is visualised in the illustration below, which also shows how it can magnify the severity of differential hypoxia. The concept has been convincingly demonstrated in an animal model by Hou et al (2015).

Diagram illustrating the concept of the “dual circulation”
The importance of the ‘dual circulation’ for an individual patient will depend on many factors, especially the degree of:
- intrinsic cardiac function
- gas exchange impairment affecting the lungs, and
- ECMO support (circuit blood flow), which influences the transition point in the aorta
The lower part of the body receives highly oxygenated blood via the femoral return cannula. Thus venous blood leaving the tissues of the lower body to enter the inferior vena cava (IVC) is likely to have O2 saturation similar to normal (e.g. SvO2 75% or higher). Most, if not all, of the blood in the IVC will be aspirated through the multistage cannula, without even reaching the right atrium, and will then circulate again via the ECMO circuit to the femoral artery. The reverse is true for the upper body circulation. Blood will return to the right atrium via the superior vena cava (SVC) and follow its natural path into the right ventricle. In the presence of pulmonary disease resulting in impaired gas
The reverse is true for the upper body circulation. Blood will return to the right atrium via the superior vena cava (SVC) and follow its natural path into the right ventricle. In the presence of pulmonary disease resulting in impaired gas exchange, this blood will remain poorly oxygenated and once ejected from the left ventricle will be distributed back to the upper body circulation. Further oxygen extraction results in markedly lower oxygen saturation in the SVC compared to the IVC.
Q8. How do you manage this condition?
The management of differential hypoxaemia involves determining what oxygen saturation you are willing to tolerate while addressing the following issues in parallel:
Treating the patient, in brief, this may involve
- Treatment of underlying lung disease (e.g. pneumonia, pulmonary oedema)
- Intubation
- Optimising ventilatory support (e.g. protective lung ventilation with increased PEEP, inspiratory time and/or FiO2)
- Increasing fluid removal (to improve pulmonary gas exchange)
- Ensure that the patient isn’t receiving unnecessary inotropes (these should be weaned as cardiac function improves)
Adjust ECMO settings
- Increasing the circuit flow need careful consideration. Initially, it may attenuate the problem however it does increase afterload and potentially contributes to ‘backwards’ LV failure, pulmonary oedema and worsening hypoxia.
Modify the ECMO configuration
- Does the patient still need V-A ECMO? Consider reconfiguration to VV ECMO
- Ameliorate the effects of the ‘dual circulation’, options for this include:
- Insert a second access cannula via jugular vein (termed a “high flow configuration”)
- Position the multistage access cannula across the IVC, RA with the tip in the SVC
- Convert to V-AV ECMO – insert an extra limb in the circuit returning oxygenated blood into the SVC via a jugular vein access as well as the femoral artery
- Replacing the femoral arterial return cannula with a return cannula in the subclavian artery via a surgical end-to-side graft
There is no uniform approach. This requires a case by case assessment guided by the experience of the treating physicians as well as institutional practice. The pros and cons of the above options will be discussed in subsequent posts.
References and Links
- Hou X, Yang X, Du Z. Superior vena cava drainage improves upper body oxygenation during veno-arterial extracorporeal membrane oxygenation in sheep. Critical Care. 19:68. 2015. [pubmed]