Notes scribed and collated by Dr Kerrianne Huynh and reviewed by A/Prof Chris Nickson
Here are the notes from the IBL-ICU discussion of the daily questions on the topic of Pharmacology and Toxicology: that took place on 4th September 2019 at ICU Junior Medical Staff Teaching.
In addition to the questions below we discussed the approach to the poisoned patient, using two cases as examples (promethazine and fampridine). To learn more:
- Approach to acute poisoning (LITFL CCC)
- Hyperthermia-associated toxidromes (LITFL CCC)
- Sedating antihistamines (LITFL Tox Library)
Q1. Thursday (12/09):
Review a patient’s drug chart with the ICU pharmacist. What are the potential drug interactions to be aware of? What are the common ICU drug interactions?
ICU patients typically receive multiple medications, increasing the likelihood of significant drug interactions. One study, found 1120 potential drug-drug interactions (pDDIs) among 275 ICU patients (!). A meta-analysis found between one and five pDDIs per ICU patients and that 58% of patients were exposed to at least one pDDI during their intensive care unit admission.
Some examples of drug interactions:
| INTERACTION | EXAMPLE |
| ALTERED GASTRIC ABSORPTION | Motility → paracetamol PPIs → altered pH → reduced azole absorption |
| INDUCE/INHIBIT ENZYMES | Azoles = ↑ tacrolimus, cyclosporine levels Carbamazepine = ↑ warfarin dose required |
| QT PROLONGATION | Macrolides, quinolones, antifungals, antiarrythmics, prokinetics, antiemetics |
| COMPLEXES AND BINDERS | Metal ions complex with enteral antimicrobials! |
| POTENTIATION OF MUSCLE WEAKNESS | Steroids and neuromuscular blockers |
| COAGULOPATHY | SSRIs + aspirin = potentiate a GI bleed |
| CRITICAL ILLNESS | Increased risk of theophylline toxicity due to ↓ metabolism SGLT2 inhibitors |
| DRUG-LAB INTERACTION | Carbamazepine falsely raises chloride |
| ↑ AUC (↑ ACTUAL BODY EXPOSURE) | Tacrolimus + azoles |
Key tip:
Be especially carefully when prescribing medications for transplant patients as drug interactions may interfere with immunosuppression and cause patient harm.
Q2. Friday (13/09):
Discuss the dosing and frequency of administration of important ICU medications with the ICU pharmacist. What factors affect the pharmacokinetics (absorption, distribution, metabolism and excretion) of these medications in critically ill patients?
Some examples:
| Factors that Influence it | |
| Absorption | Reduced gastric motility and perfusion (noradrenaline, shock) Parenteral routes: poor perfusion, IM route may have less blood supply and be affected by adiposity or oedema Extracorporeal circuits (adsorption) |
| Distribution | Greater volume of distribution in patients with vasodilatory shock and third spacing (hydrophilic drugs, gentamicin) Degree of protein binding Acid base abnormalities Extracorporeal circuits – fentanyl is the classic example of a drug that binds to the circuit |
| Metabolism | Degree of metabolism depends on the patient population (paeds vs adults vs obesity vs elderly vs pregnancy) Liver dysfunction (antibiotics, induced enzymes) Temperature – reduced metabolism at hypothermic states (cisatracurium: spontaneous Hoffman degradation) Hypermetabolic states (trauma, burns, sepsis) |
| Excretion | Renal dysfunction Increased metabolic states may excrete more quickly There is increased elimination in those on RRT and those with augmented renal clearance (young trauma and burns patients can have massive increased drug clearance early in ICU stay) Surgical patients with high output stomas or drains (can be like having an extra kidney!) |
Learn more (LITFL CCC):
Q3. Saturday (14/09):
Discuss a patient who may benefit from therapeutic drug monitoring (TDM). When should we perform TDM – which drugs and in which patients? What practical issues affect TDM?
We discussed some common ICU examples of TDM:
- Antimicrobials (e.g. gentamicin and vancomycin, posoconazole )
- Tacrolimus
- Anti-epileptics
Learn more (LITFL CCC):
Q4. Sunday (15/09):
Review a patient who has renal impairment (+/- renal replacement therapy). How does it affect the dosing and timing of their medications? How does renal replacement therapy alter this?
Some tips in renal impairment:
- Loading doses are the same
- Reduce doses for drugs that are renally cleared (e.g. enoxaparin, many antimicrobials, oral hypoglycemics, many opioid drugs, )
- Reduce the dose
- Increase the interval between doses
- Some drugs need to be increased! (frusemide)
- Remember to restore these doses to normal when on continuous renal replacement therapy OR give drugs AFTER intermittent haemodialysis
- Drug monitoring may help!
- If in doubt, ask your pharmacist and look it up
- check local drug guidelines (e.g. Alfred Health intranet)
- Therapeutic Guidelines – Antibiotics has a section on dose adjustment in renal insufficiency
Q5. Monday (16/09):
Review a patient with complex analgesia needs. Discuss the advantages and disadvantages of different pharmacological therapies for this patient? (e.g. opioids, partial opioid agonists (such as buprenorphine, tramadol/ tapentadol), paracetamol, NSAIDS, ketamine, and lignocaine infusions)
This is a complex topic! A brief overview is provided by this table:
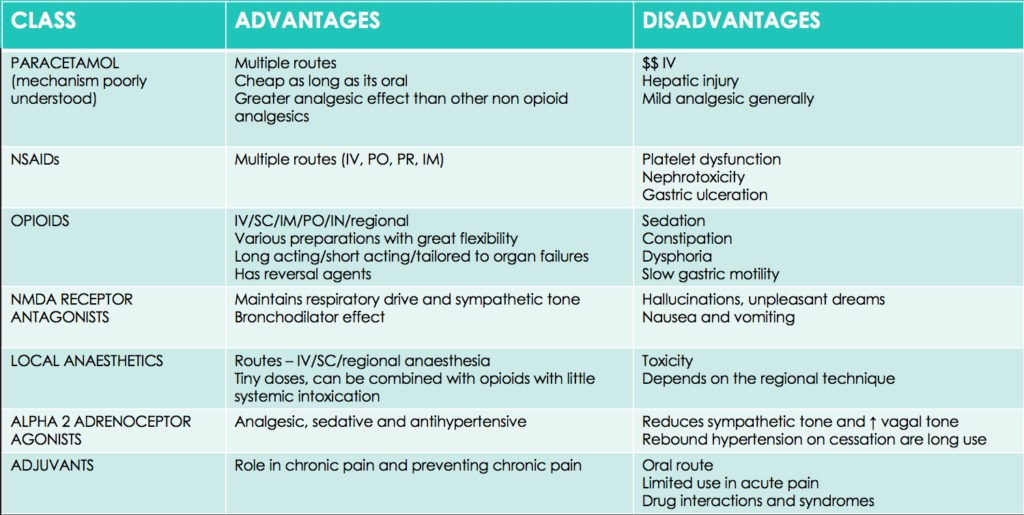
Some points highlighted in our discussion:
- ICU patients often have (at least relative) contra-indications to NSAIDs (e.g. transplant patients, renal impairment, risk of peptic ulcer disease, bleeding risk, poor wound healing)
- Infusions increasingly used in ICU, especially for refractory pain include:
- clonidine
- ketamine
- lignocaine
- We are in the midst of opioid crisis and tramadol is the root of all evil 😉
Learn more:
- Acute Pain Management: Scientific Evidence (5th Edition) (pdf)
- Tox & Hound: Tramadont (by David Juurlink)