Everything ECMO 041: Loss of arterial pulsatility
Authors: Dr Kenji Morimoto, Dr Rob Paul
Reviewer: A/Prof Chris Nickson
A 42 year-old male had an out-of-hospital cardiac arrest (OOHCA) and ECPR was initiated in the community.
The patient had an initial rhythm of ventricular fibrillation (VF) and is now in sinus rhythm, since ECMO was initiated.
The ECMO configuration is femoral-femoral veno-arterial (VA)- ECMO, with a 15Fr return cannula and 19Fr multistage access cannula.
The patient is requiring vasopressor support to maintain a mean arterial pressure (MAP) > 65 mmHg.
You took the handover from ECMO transport team (1.5hr post ECMO initiation) and saw this on the monitor:
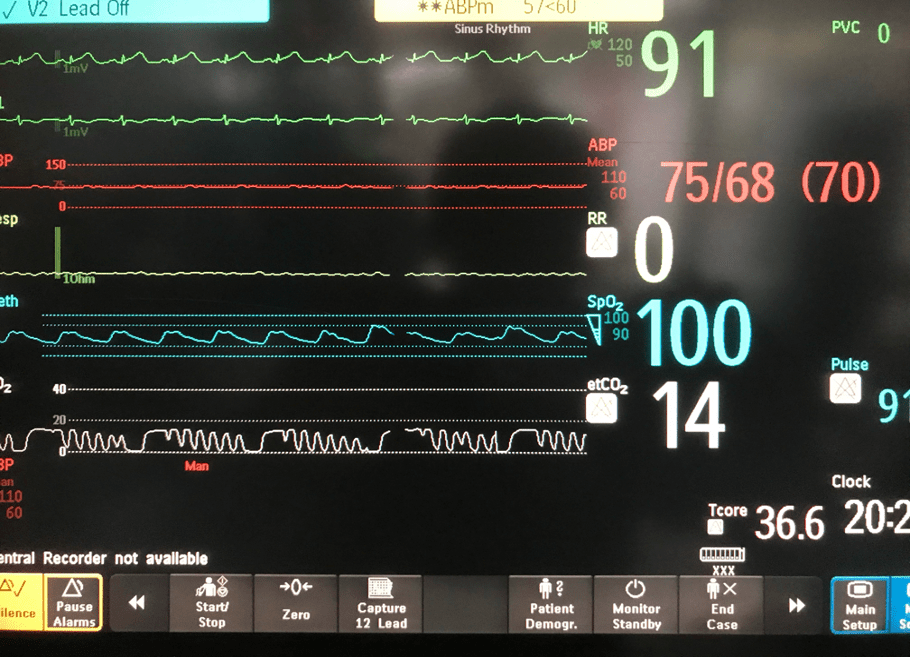
Q1. What are findings on the monitor?
Key findings include:
- Sinus rhythm (91/min)
- reasonable MAP (70 mmHg)
- Flattened arterial line waveform (pulse pressure (PP) = 7 mmHg)
- low end-tidal CO2 (etCO2 = 14 mmHg)
- Oxygen saturation (SpO2) = 100%
- Respiratory rate = 0/min (likely that sensor not detecting low volume mechanical ventilation)
Q2. What does the flattened arterial line trace mean?
This is loss of arterial pulsatility (pulse difference < 10-15 mmHg).
Arterial pulsatility reflects native cardiac function on ECMO support. The loss of arterial pulsatility means loss of native cardiac function. Maintenance of native cardiac function is a primary aim of VA ECMO support, as this promotes recovery and prevents further complications.
Patients receiving VA ECMO who have loss of pulsatility typically still have a supported circulation, at least in the short-term. Whereas loss of pulsatility in veno-venous (VV) ECMO means cardiac arrest!
Note that in extreme loss of pulsatility, an accurate MAP will still be present on the arterial trace in the absence of systolic and diastolic measurements.
Q3. What are the consequences of this?
Thrombosis
- Results from stagnation or low flow across the left ventricle (LV) and aortic root
- Echocardiographically this may manifest as spontaneous echo contrast (SEC) which may progress to manifest thrombus
- Consequences
- Massive intracardiac thrombosis is generally lethal
- Thromboembolism with potential cerebral, limb, kidney or gut ischaemia / infarction
Increased LV filling pressure/ High LV load
- Loss of LV ejection (forward flow) can increase the risk of pump driven LV distention (LV Failure).
- Consequences
- Impaired coronary perfusion and further ischaemic damage to the myocardium
- Mitral regurgitation with resultant left atrial hypertension, pulmonary oedema, pulmonary capillary rupture and haemorrhage.
- See Everything ECMO 020: “Pink fluid coming up the tube”.. ECMO, LV distention, and more!
Q4. What are the causes of the flattened arterial line wave form?
Causes of loss of pulsatility on the arterial line trace include:
- Artifact
- Inadequate preload (e.g. excessive VA ECMO flows, haemorrhage)
- Mechanical obstruction (e.g. tamponade, tension pneumothorax, right ventricular outflow tract (RVOT) or LVOT obstruction, and pulmonary embolism). Intrathoracic bleeding causing cardiac compression is a common cause immediately following intrathoracic surgery.
- Arrhythmias
- LV failure
- Poor LV contractility, e.g. ischaemia / infarction
- Excessive afterload: typical MAP target 65-70 mmHg
- Valvular regurgitation: Aortic or Mitral regurgitation
Q5. How should you assess this?
Clinical assessment of loss of arterial pulsatility should consider following causes:
- Artifact: check for damping of arterial waveform and palpable peripheral pulses. Check the pressure bag is inflated, check the tubing (e.g. for air bubbles, or wrong tubing), and flush the arterial line (e.g. to exclude clot). Replace arterial line if required.
- Arrhythmia: exclude non-perfusing rhythms
- Console: check flow and pump speed setting. Assess for signs of access insufficiency and perform dynamic assessment
- Haemoglobin: look for downward trend on arterial blood gas (ABG) and full blood count (FBC); exclude clinically apparent bleeding (retroperitoneal, pleural, gastrointestinal)
- Tension pneumothorax: palpate, Auscultate and consider chest x-ray (CXR)
- Tamponade: measure central venous pressure (CVP) (and pulmonary pressures if a pulmonary catheter is in situ), assess for access Insufficiency, and perform bedside echocardiography
- Pulmonary oedema: check endotracheal aspirates for frothy secretions +- haemorrhage, look for pulmonary oedema on CXR.
Echocardiographic assessment is essential, looking for features of:
- Cannula position (also check on CXR)
- Tamponade
- Right heart failure
- RV: size, function, compression/obstruction (e.g. in severe RVOT hypertrophy), SEC or thrombosis
- valves: valvular regurgitation (may be continuous)
- Left heart failure
- LV: size (LVEDD), function, compression / obstruction, SEC or thrombosis
- valves: mitral or aortic regurgitation (may be continuous), frequency and adequacy of aortic valve opening
Note: In ECPR and more severe forms of acute cardiac failure, it is common to lose pulsatility immediately after commencing VA ECMO. Cardiac ultrasound assessment is usually performed around this time.
Additional eFAST assessment may be performed concurrently where appropriate (e.g. assessing for pneumothorax (loss of lung sliding, presence of lung point) and haemothorax / substantial intraperitoneal fluid).
Q6. What is the relation between ECMO flow and native cardiac output?
VA ECMO supports the systemic circulation when native cardiac output is insufficient, by reducing the LV preload and delivering the blood to the systemic circulation.
However, high flow from a VA ECMO circuit impairs cardiac performance. Increasing ECMO flows worsens LV stroke volume, ejection fraction, and cardiac output, while increasing LV stroke work (Ostadel et al, 2014) and increases pulmonary venous congestion/ pulmonary capiullary wedge pressure (PCWP) (Disckstein and Bacchetta, 2015).
Optimum VA ECMO support is a balance of systemic perfusion delivered by the VA ECMO circuit and minimising native LV dysfunction. A rough guide should be for the VA ECMO circuit to provide approx. 60–80% of the predicted cardiac output allowing for the remaining 20–40% to pass through the lungs and heart (Chung et al, 2014).
Q7. You are concerned that your patient has excessive ECMO flows, so you reduce the RPM until the flow is 2.5 litres. How will you assess the adequacy of the ECMO support?
Clinical assessment:
- Neurological function
- Renal function (urine output, serum creatinine)
- Liver (synthetic and metabolic function)
- Peripheral warmth & skin perfusion
- GI function
- Pulse pressure (>10-15 mmHg)
Ideally, investigations would show:
- Lactate <2.0 mmol/L
- Mixed venous O2 sat >60%
- Renal and liver function bloods stable/improving
- ETCO2 >20mmHg
- Echo: LV normal size, Aortic valve opening with each systole, no LV thrombus or spontaneous echo contrast.
Unfortunately, the echo findings in your patient are not what you hoped…
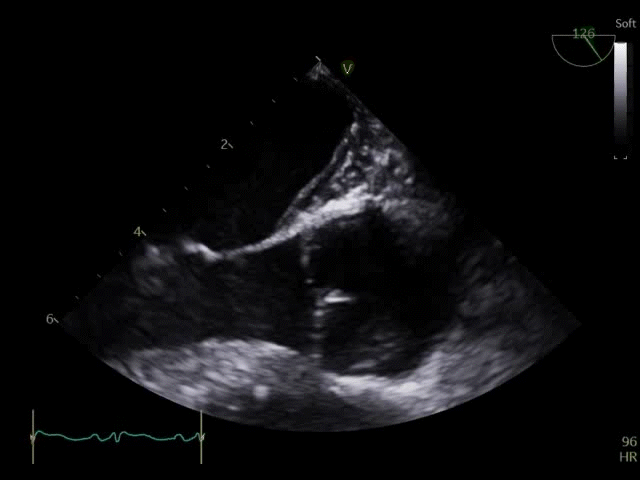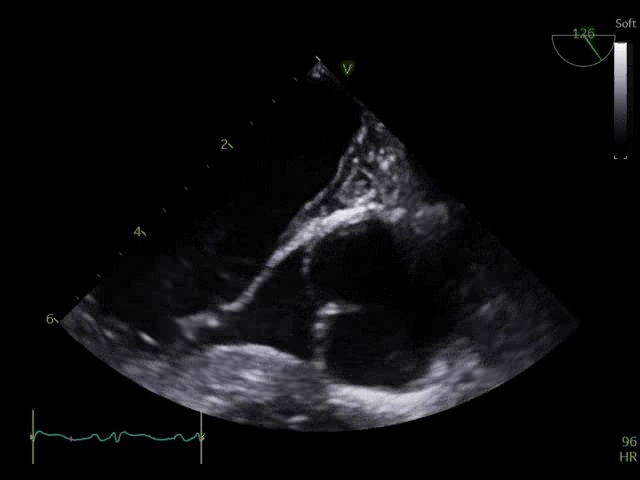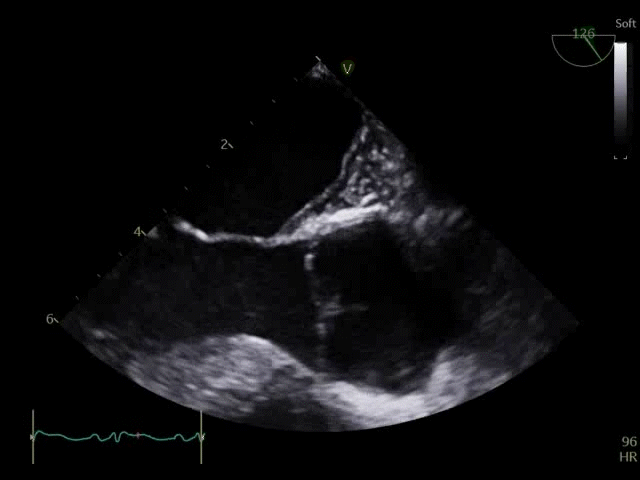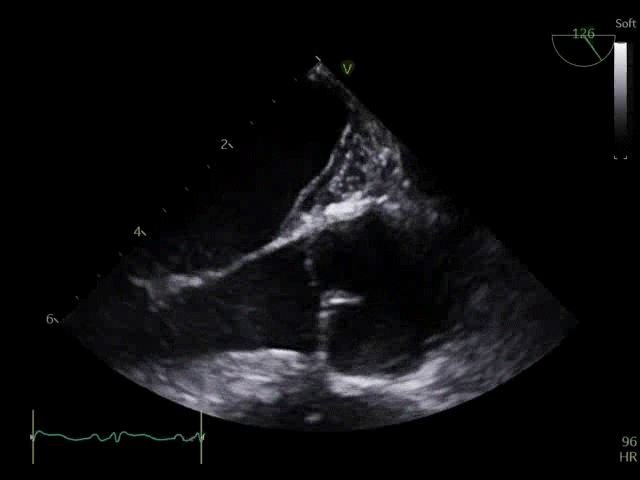
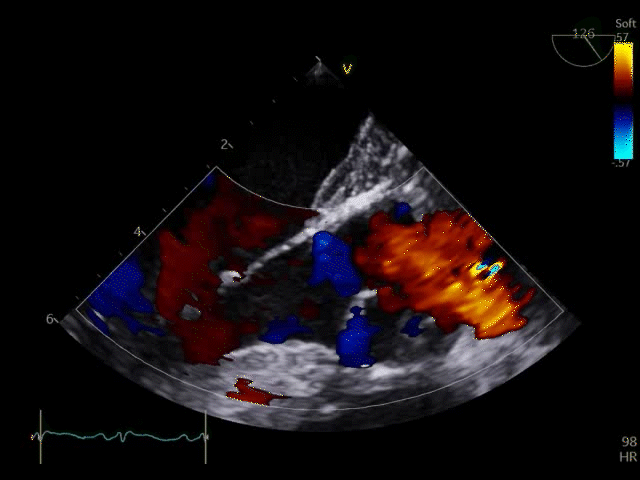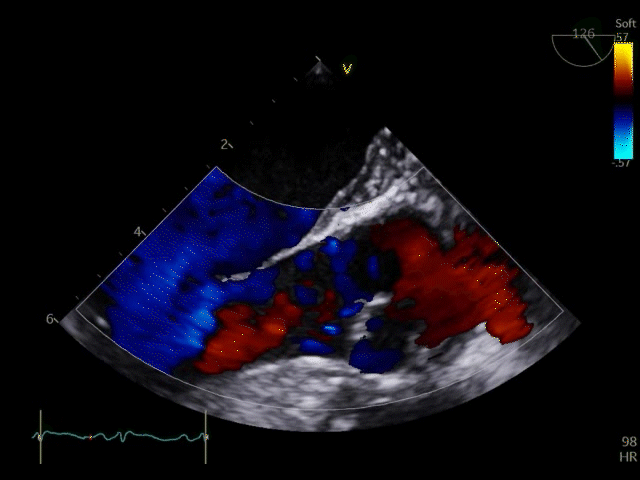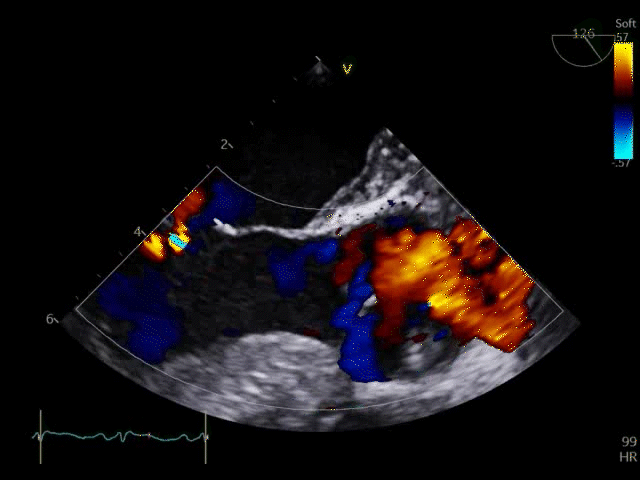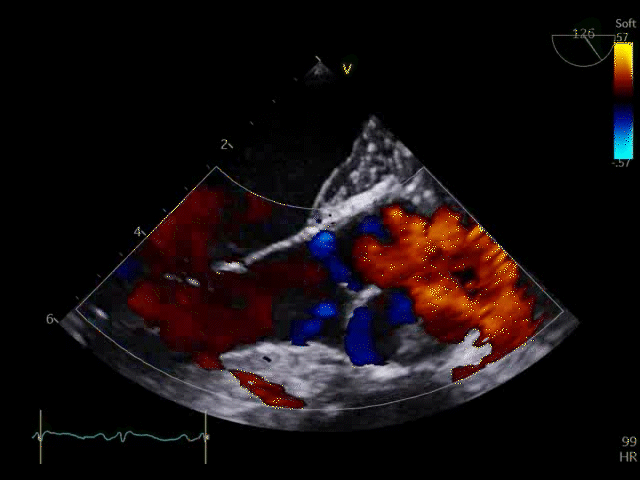
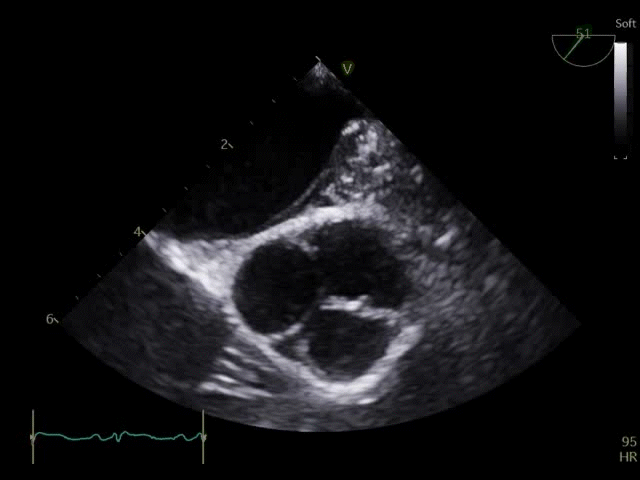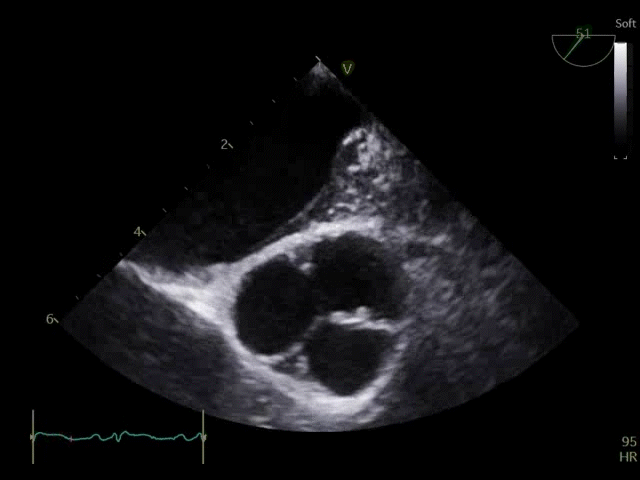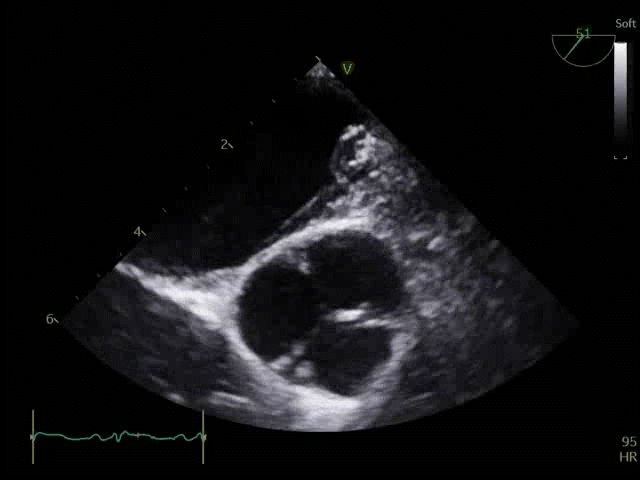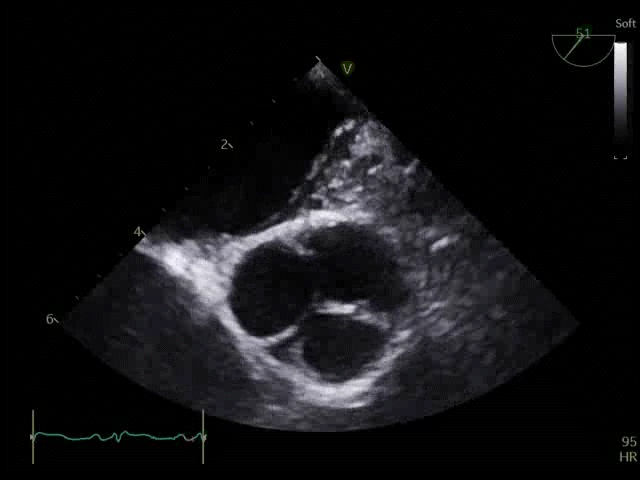
Q8. Transoesophageal Echocardiography shows the images below. What is the key finding and what does it mean?
TOE shows a non-opening aortic valve, which means no flow through LVOT (i.e. no forward flow) and contributes to LV distention and high LVEDP.
Q9. How can this be managed?
Medical management should focus on addressing the key issues contributing to poor pulsatility. In the absence of pulmonary oedema, with adequate systemic perfusion and MAP, managing inadequate preload and / or excessive afterload may resolve the loss of pulsatility. However, medical management is not a comprehensive or longterm strategy, and prevents extubation, where recovery of LV function is not anticipated or there is significant risk of progressive LV distention syndrome.
Excessive afterload
- review MAP targets
- lower MAP of 60-70mmHg is usually appropriate
- Consider inodilators / vasodilators
- Note: inotropes may exacerbate LV distension if LV is non-responsive to inotropes and effect of inotropes to primarily to increase RV output
- PEEP application
- High positive end-expiratory pressure (PEEP of 15 to 25 cmH20) is usually appropriate
- Aggressive afterload control cannot be underestimated in these situations.
Inadequate preload
- consider volume loading (including blood / blood products).
- Note: LV failure with LV distention syndrome is usually associated with a “sucked down” RA and IVC. Volume loading will not correct this problem. The “need” for volume loading in VA ECMO should always prompt a search for LV failure and bleeding.
Poor contractility
- treat underlying cause (e.g. angiography for ischaemia)
- Inotropic support for maintenance of pulsatility (see excessive afterload above)
- optimize temperature, acidosis, pO2 and pCO2
Arrhythmia
- aim for K+ > 4.5 and Mg > 1 (or higher) in ventricular arrhythmias
- consider amiodarone / direct current cardioversion (DCR)
- β blockade may be instituted after senior consultation
- Consider cardiology referral.
RV / LV failure
- consider inodilators or constrictors (e.g. milrinone, adrenaline) as dictated by MAP, with pulmonary vasodilators (e.g. iNO) if required.
Optimise VA ECMO flow
- Note: where RV systolic function is better than LV systolic function reducing pulmonary blood flow by increased ECMO blood flow can be effective. However, the afterload on the LV needs to be controlled to MAP target with systemic vasodilators as required to encourage forward flow through the LVOT and prevent LV distention.
Mechanical obstruction
- Surgical intervention is required for loss of pulsatility due to mechanical obstruction from causes such as pericardial tamponade.
Q10. Despite these measures, pulsatility remains poor. How can the consequences of loss of pulsatility be managed?
As discussed earlier, the two major consequences are:
- thrombosis
- LV distention syndrome
In addition to the medical management already discussed, measures to combat the consequences of loss of pulsatility are thus:
- Anticoagulation
- increased anticoagulation, particularly in the setting of SEC or thrombus, should be considered in the context of other clinical priorities (e.g. haemostasis).
- Mechanical LV decompression interventions should be considered early for cases with significant LV distention and for cases that are expected to progress. Options are discussed in Everything ECMO 020 and in the Alfred ICU ECMO guidelines – LV Distention Syndrome, they include:
- Trans-apical surgical LV vent cannula
- Trans-aortic LV ‘pigtail’ catheter
- Impella®
- Pulmonary arterial drainage via single lumen cannula
- Pulmonary venous drainage in conjunction with central VA ECMO cannulation
References
- Alfred ICU ECMO Guidelines. Loss of arterial pulsatility. ECMO.ICU. [Accessed 12 August 2022] Available at URL: https://ecmo.icu/va-ecmo-loss-of-arterial-pulsatility/ (thisoverall approach describes in this blogpost is based on this guideline)
- Dickstein M, Bacchetta M. The Impact of VA ECMO on LV Function. J Heart Lung Transplant 2014; 33:S246 [article]
- Napp LC, Kühn C, Bauersachs J. ECMO in cardiac arrest and cardiogenic shock. ECMO bei Herz-Kreislauf-Stillstand und kardiogenem Schock. Herz. 2017;42(1):27-44. doi:10.1007/s00059-016-4523-4 [article]
- Ostadal P, Mlcek M, Gorhan H, Simundic I, Strunina S, Hrachovina M, et al. Electrocardiogram-synchronized pulsatile extracorporeal life support preserves left ventricular function and coronary flow in a porcine model of cardiogenic shock. PLOS ONE; 2018;13(4):e0196321. [article]

VentriFlo Inc has a pulsatile pump that can deliver a true pulse through a standard oxygenator.
VentriFlo.com