Author: Dr. Vinodh Nanjayya
Peer reviewer: Dr. Aidan Burrell
Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region — Case Series. New Eng J Med 2020. Published online March 30, 2020. doi: 10.1056/NEJMoa2004500
In the New England Journal of Medicine, Bhatraju et al. published a multicenter observational study (a case series) from Seattle, United States of America, which looked at the outcome of patients admitted to ICU for COVID-19 (See Figure 1)
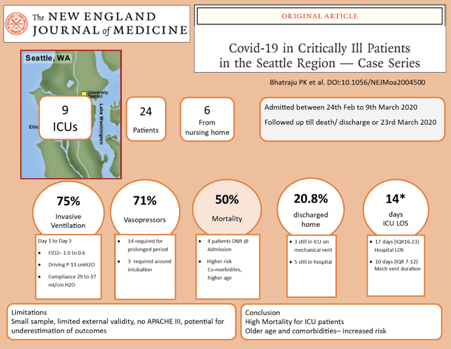
*ICU LOS, Hospital LOS and mechanical ventilation duration are only for the survivors.
The study included 24 patients from nine ICUs admitted between 24th February and 9th March 2020, and the researchers followed the patients until discharge/death or the censor date of 23rd March 2020. They included patients between 23-97 (Mean 64) years of age, 61% males, and most of the patients had co-morbidities like diabetes mellitus. The symptoms preceded hospital admission by 3-11 (mean 7) days, and 25% of the patients were nursing home residents. Cough and shortness of breath were the main symptoms at admission and lymphopenia was the common abnormality on lab investigations. Chest X-ray at the time of ICU admission showed bilateral pulmonary opacities. CT Scan (conducted in 5 patients) showed bilateral ground glass opacities and pulmonary nodules, and echocardiography (performed in 9 patients) did not show any new left ventricular dysfunction.
Eighteen patients (75%) received mechanical ventilation (MV). The median FiO2 and compliance improved from 1.0 to 0.6 and from 29 ml/cm H2O to 37 ml/cmH2O, respectively, from day 1 to day 3. In contrast, the driving pressure remained between 12 to 13 cmH2O during the first three days. Seventeen patients (71%) had hypotension requiring vasopressor support and none of these patients had evidence of secondary bacterial/fungal infections. At the censor date, 12 patients (50%) had died, 5 (20.8%) patients were discharged from hospital, 4(16.6%) patients were discharged from ICU, and 3 (12.5%) patients were still in ICU on MV. Patients with co-morbidities had a higher risk of death. The median duration of mechanical ventilation and ICU length of stay among survivors were 10 (IQR 7-12) days and 14 (IQR 4-17) days, respectively.
Discussion
This study provides the one of the first reports of ICU epidemiology of COVID-19 in the United States. The study demonstrated that despite aggressive ventilation measures, the mortality remained high at around 50% (this mortality is lower compared to some of the previously published studies [See Table 1 for mortality from various studies]). As a developed Western country, data from this study may be more generalizable to Australia than reports that have come from other countries, such as China (although the inclusion of nursing home residents is uncommon in Australian practice). There are several limitations to the study. The small sample size makes it difficult to draw any firm conclusions from this data. The authors do not provide a CONSORT diagram, APACHE scores or any details about renal failure. There are missing data, and about 29% of patients were still in the hospital at the time of censoring of the data. Therefore, this study could be underestimating the mortality and other outcomes. Further large, multi-centre observational studies with complete follow-up are needed to improve our understanding of the ICU epidemiology of COVID-19.
Table 1. Comparison of mortality between various ICU studies published till 9th April 2020.
| Study Name | N | ICU mortality |
| Bhatraju PK et al. NEJM | 24 | 50% |
| Arentz M et al. JAMA | 21 | 67% |
| Zhou F et al. Lancet* | 50 | 72% |
| Yang X et al Lancet Respir Med | 52 | 61.5% |
| Wang D et al JAMA | 36 | 16% |
| Huang C et al Lancet | 13 | 38% |
| Graselli G et al JAMA | 1591 | 26% |
*This is the only study which had the complete follow-up of the patients till death or discharge from hospital. Rest of the studies had patients in the ICU at the time of censoring. Hence, the mortality will be an underestimation.
Further Reading
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. Published online March 19, 2020. doi:10.1001/jama.2020.4326
- Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region — Case Series. New Eng J Med 2020. Published online March 30, 2020. doi: 10.1056/NEJMoa2004500
- Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020. Published online April 6, 2020. doi: 10.1001/jama.2020.5394
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; S2213-2600(20)30079-5. Published online February 24, 2020. doi:10.1016/S2213-2600(20)30079-5
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3