Author: Arun Ilancheran
Peer reviewers: Aidan Burrell, Chris Nickson
Everything ECMO 032
A 32 year-old female is transferred to your ECMO centre. She is currently Day 3 in ICU with severe community acquired pneumonia (CAP) and bilateral lung infiltrates, and is requiring high vasopressor support. She has ongoing hypoxia despite maximally optimised ventilation strategies and proning. Her saturations (SpO2) are 86% with a PaO2 of 50 mmHg on a Fi02 of 1.0 and a PEEP of 20 cmH20.
Your team decides to put her onto V-V ECMO. She is put on femoral-femoral V-V ECMO with a 21F multistage access and 19F return.
Relevant Pre-ECMO full blood count and coagulation profile results are:
INR 1.3
APTT 30s
Fibrinogen 2.5 g/L
D-dimer 1.7 mcg/mL
Haemoglobin 110 g/L
Platelets 150 x 10E9/L
She is commenced on 500 U/h of Heparin (no bolus) to run concurrently with her ECMO circuit to systemically anti-coagulate her with an APTT aim of 50-70s.
Q1. Explain the rationale for heparin anticoagulation on ECMO.
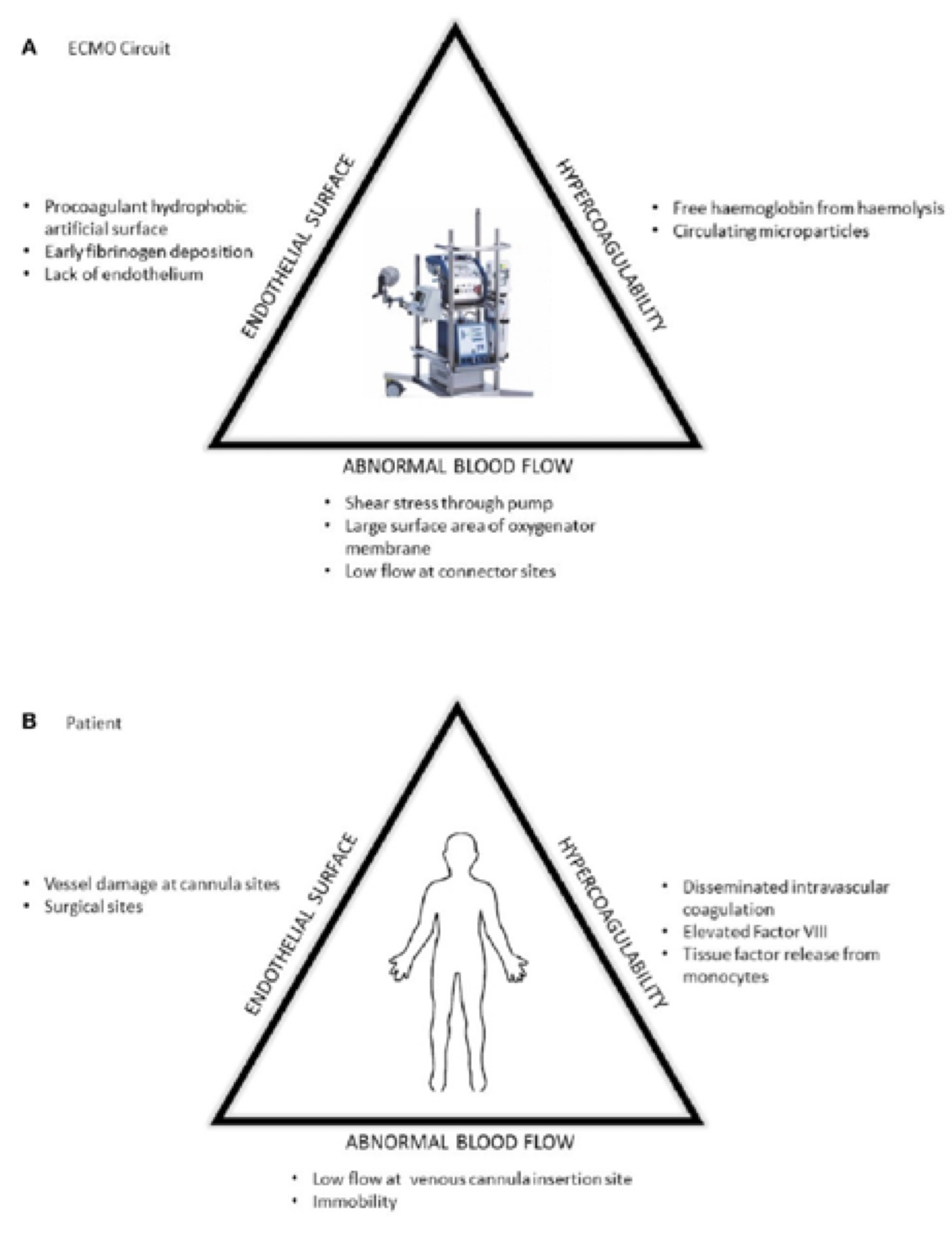
Heparin anticoagulation is used in ECMO patients to prevent thrombosis, both of the circuit and the patients’ underlying illness. ECMO related venous thromboembolic complications may occur in up to 85% of patients, and may be at least partly dependant on anticoagulation regimens.1,2 Unfractionated Heparin is the most widely used systemic anticoagulant used in Extracorporeal Life Support (ECLS). However, unfractionated heparin only inhibits thrombin after it is formed. It does not prevent thrombin formation nor does it inhibit thrombin already bound to fibrin.3
The reasons for the pro-thrombotic states include factors such as contact pathway activation, haemolysis and free haemoglobin, vessel injury at cannulation, micro-thrombi formation and pre-existing systemic inflammation in patients due to the disease process.4
A week later, the patient is on high-flow VV-V ECMO with saturations of 94% on 4.5L of ECMO flow at 3300 RPM. Her fresh gas flow (sweep gas) is at 6L/min. Her HR is 110 /min, BP is 90/55 mmHg on 10 mcg/min of Noradrenaline. Her PaO2 is 80 mmHg. She is receiving 1500 units/h of Heparin.
Relevant laboratory tests and circuit transmembrane pressure (TMP) gradient are:
INR 1.5
APTT 55 s
Fibrinogen 1.6 g/L
D-dimer 12 mcg/mL
Haemoglobin 75 g/L
Platelets 90 x10E0/L
Plasma free Hb = 0.5 g/dL
Pre-oxygenator PaO2 100 mmHg
Post oxygenator PaO2 150 mmHg
Transmembrane pressure (TMP) gradient – 50 mmHg
Q2. Interpret the above findings. What is happening?
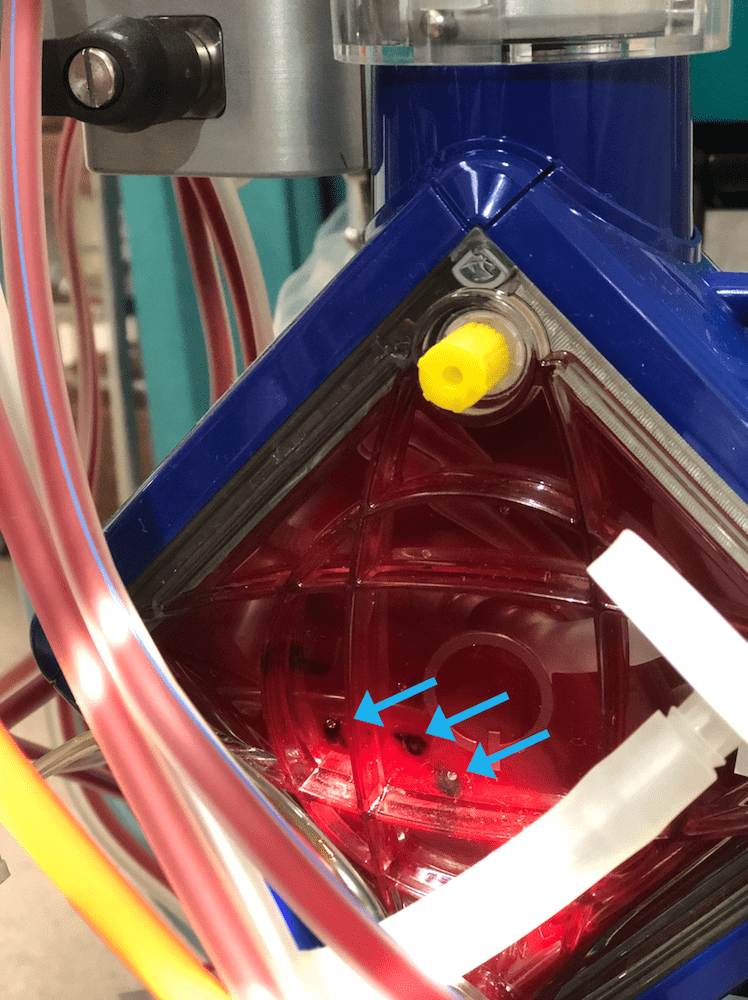
Oxygenator thrombosis The APTT is therapeutic (lower end of the therapeutic range) but she is hypofibrinogenaemic with a markedly raised D-dimer, low platelets and a raised INR. The raised TMP (normally <10 for each L/min of blood flow), raised plasma free Hb and low post oxygenator gases with these above coagulation markers are suspicious for significant oxygenator thrombosis. Visual inspection of the oxygenator reveals this (Figure 1): Figure 1. Blue arrows indicate “clots” in the oxygenator
Q3. What are the mechanisms that result in hypofibrinogenaemia following ECMO initiation?
Fibrinogen is an essential substrate for the formation of a fibrin-based blood clot. Fibrinogen is enzymatically converted to fibrin by thrombin.
A reduction in circulating fibrinogen post ECMO commencement can be due to:
- Consumption, due to:Adhesion to biosurfaces and loss from circulation5
Accelerated conversion of fibrinogen to fibrin due to pro-inflammatory state caused by complement activation and polymorphonuclear (PNM) interactions - Hyperfibrinolysis, due to (see also Q4):
An over-activation of an essential system to maintain homeostasis with degradation of fibrin complexes once the injury is resolved.
A rise in tPA (tissue plasminogen activator; promoting activation of plasminogen to plasmin to break down fibrin products) and PAI-1 levels hours after ECMO initiation6.
- Sepsis
Q4. Why are some ECMO patients prone to hyperfibrinolysis?
The mechanism of hyperfibrinolysis in ECMO patients is currently poorly understood. The release of tPA is stimulated by thrombin, hypoxia, vasopressin use and histamine release. A multitude of these factors are at play in the ECMO patient.
Q5. What laboratory tests are used to confirm hyperfibrinolysis?
- D-dimer
- Fibrinogen levels
- Fibrin degradation products
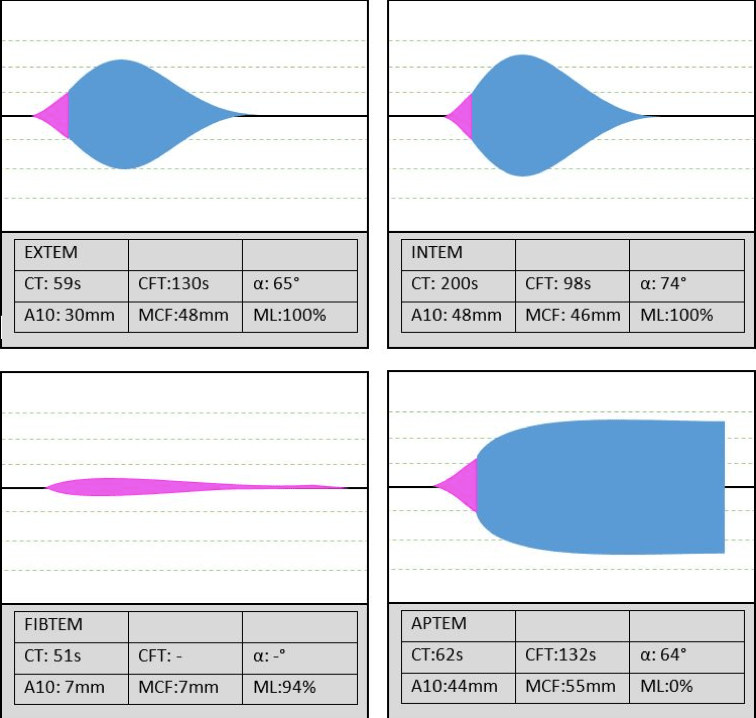
- Whole blood point of care tests with viscoelastic assays – TEG and ROTEM. These are extremely operator dependant tests that might not be available at every institution. The interpretation of the graphs are wholly dependant on the user and their inter-rater reliability has shown to be poor.
| Normal Range | Moderate Abnormal | Severe Abnormal | |
| D-dimer | 0-0.3 mcg/mL | >2-15 mcg/mL | >15 mcg/m: |
| Fibrinogen | 2.1-4.3g/L | <1.5g/L | <1.0g/L |
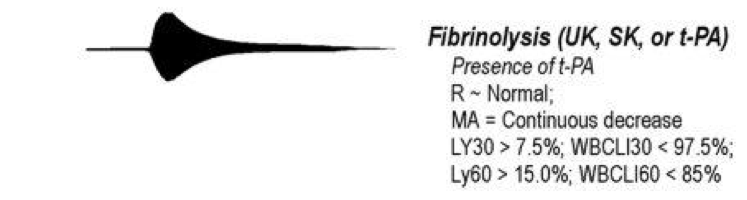
Figure 3. Appearance of Hyperfibrinolysis on TEG (above)(see reference 7 to learn more)
4 hours later, you repeat the bloods. The D-dimer and Plasma-free Hb are rising and the fibrinogen is lower. You immediately gather the team to do an urgent circuit change (see Everything ECMO 012 —Turn and face the circuit… ch-ch-change it!)…
References
- Cooper E, Burns J, Retter A, Salt G, Camporota L, Meadows CI, et al. Prevalence of venous thrombosis following venovenous extracorporeal membrane oxygenation in patients with severe respiratory failure. Crit Care Med. (2015) 43:e581–4. doi: 10.1097/CCM.0000000000001277 [pubmed]
- Menaker J, Tabatabai A, Rector R, Dolly K, Kufera J, Lee E, et al. Incidence of cannula-associated deep vein thrombosis after veno-venous extracorporeal membrane oxygenation. ASAIO J. (2017) 63:588–91. doi: 10.1097/MAT.0000000000000539 [pubmed]
- Doyle AJ, Hunt BJ. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. ELSO Guideline. Accessed 15 April 2019. Available at URL: https://www.elso.org/portals/0/files/elsoanticoagulationguideline8-2014-table-contents.pdf
- Passmore MR, Fung YL, Simonova G, Foley SR, Diab SD, Dunster KR, et al. Evidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation support. Crit Care (2017) 21:1-9. doi: 10.1186/s13054-017-1788-9 [pubmed]
- McVeen RV, Lorch V, Carroll RC, Goldberg L, Keszler M, Podlasek S, et al. Letter to the editor: Changes in fibrinolytic factors in newborns during extracorporeal membrane oxygenation (ECMO). Am J Hematol. (1991) 38:254–55. [pubmed]
- Doyle AJ, Hunt BJ. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. (2018) Front Med. 5:352. doi: 10.3389/fmed.2018.00352 [pubmed]
- Nickson CP. Thromboelastogram (TEG). Critical Care Compendium. LITFL.com. Accessed 15 April 2019. Available at URL: https://litfl.com/thromboelastogram-teg/
- Yartsev A. Intepretation of abnormal ROTEM data. DerangedPhysiology.com. Accessed 15 April 2019. Available at URL: https://derangedphysiology.com/main/required-reading/haematology-and-oncology/Chapter%201.2.0.1/intepretation-abnormal-rotem-data
All case-based scenarios on INTENSIVE are fictional. They may include realistic non-identifiable clinical data and are derived from learning points taken from clinical practice. Clinical details are not those of any particular person; they are created to add educational value to the scenarios.