Author: Shir Lynn Lim
Peer reviewers: Aidan Burrell, Arne Diehl, Chris Nickson
Everything ECMO 020
A 39-year-old male was commenced on peripheral fem-fem VA ECMO for cardiogenic shock following a late presentation of anterolateral STEMI. His ECMO flows had been stable at 3L/min. On the second day, he becomes progressively more difficult to ventilate and you notice a large volume of pink fluid coming up his endotracheal tube.
A chest x-ray is obtained:
Q1. What are the potential causes?
The X-ray shows an enlarged heart, four quadrant infiltrates, and a multistage access cannula with the tip positioned in the superior vena cava.
Potential causes include acute pulmonary oedema. Other possibilities include primary lung pathologies, such as capillary leak syndromes, aspiration, or possibility ARDS with a high sputum/fluid load.
Given the clinical context and with fluid coming up the ETT, the picture is most suggestive of inadequate left ventricular (LV) unloading and progressive LV failure.
Q2. How will you confirm the diagnosis?
You arrange an urgent echocardiogram.
You look for the following features to confirm your diagnosis of inadequate LV unloading: distended and non-contractile LV, aortic valve opening only intermittently or not at all, bowing of the inter-atrial septum towards the right and severe mitral regurgitation.
Not all features LV distension are present in acute cardiogenic shock secondary to myocarditis or acute myocardial infarction, as LV dimensions remain within normal limits and significant mitral regurgitation may be absent.
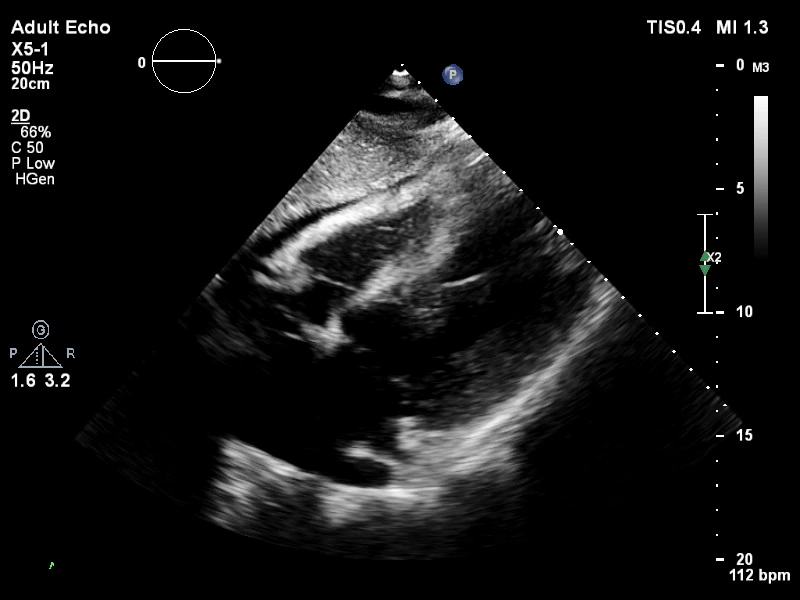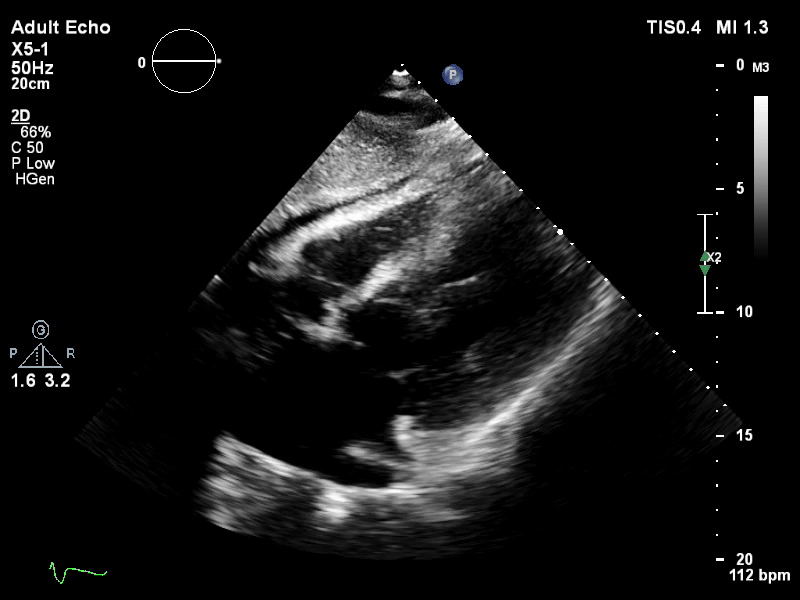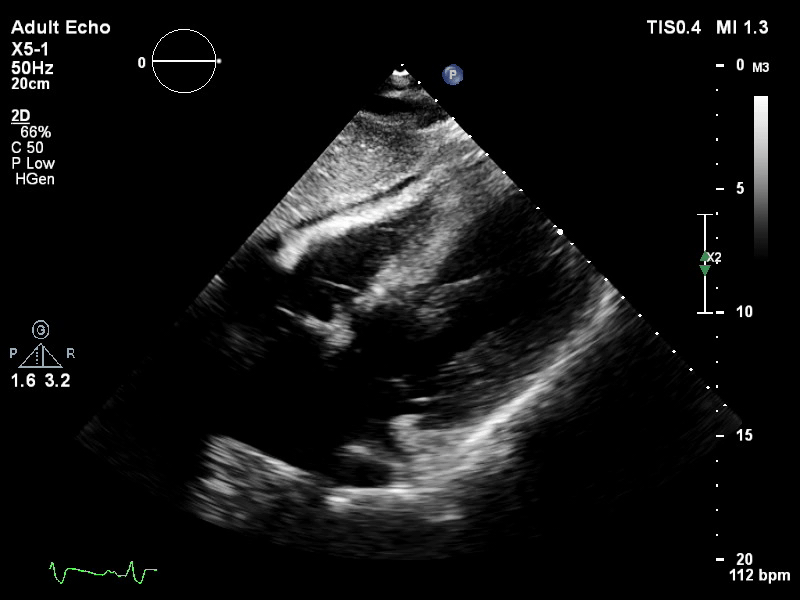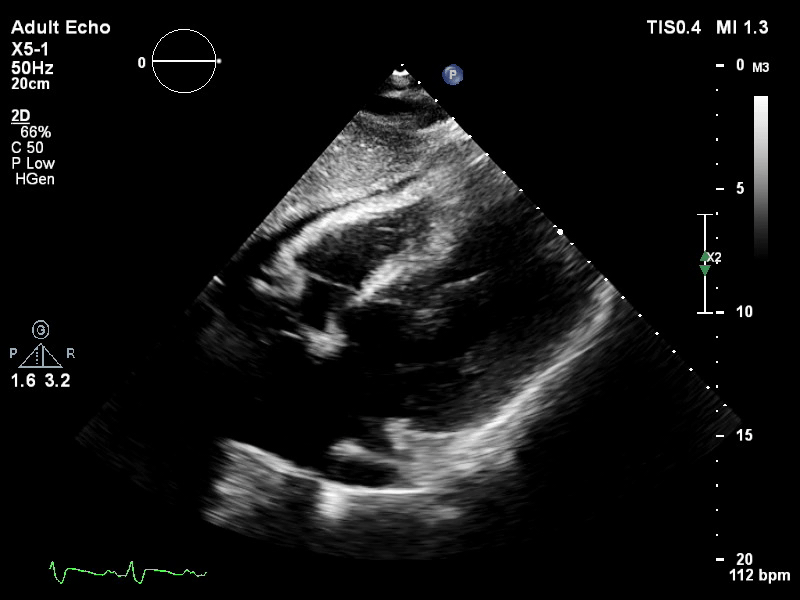
TTE subcostal view of distended left ventricle on VA ECMO.
Q3. What are the mechanisms for LV distension?
VA ECMO supports the heart by draining venous blood which leads to a reduction in LV preload, and maintains aortic blood flow which can improve coronary perfusion. However cardiac function may be impaired by VA ECMO through several other mechanisms. There remains ongoing blood return to the LV via the bronchial, thesbesian and pulmonary circulations which are not captured by the circuit. VA ECMO also increases the afterload on the left ventricle via its return cannula pumping blood retrograde into the aorta. In each individual, different factors play a greater or lessor importance, and in a subgroup, inadequate LV unloading results. This can lead to cessation of native blood flow through the heart, LV stasis and LV distention.
Any aortic valve incompetence prior to ECMO tends to worsen with retrograde aortic flow as well, and this also contributes to the reduction in forward flow and left ventricular volume overload and distension.
Q4. What impact does LV distension have on the patient?
- The distending LV causes a rise in intraventricular pressure and wall tension which can lead to increased oxygen consumption and can worsen ischaemic damage.
- Increased shear stress to the infarct area could potentially result in ventricular free wall rupture.
- Stasis in the LV can result in intra-cardiac thrombus formation.
- Sustained LV distension can lead to severe pulmonary oedema and haemorrhage which can make ventilation almost impossible.
Q5. What can be done acutely?
Interventions include:
- Increasing PEEP will reduce wall stress on the LV and may further reduce LV preload. It may have to be very high (ie >20cmH20) in order to overcome the extreme elevation in LAP and pulmonary oedema. Clamping the ETT is generally not recommended but has been described in the past to prevent uncontrollable pulmonary oedema from blocking up the ventilator circuit.
- Increasing inotropic support improves myocardial contractility and promotes LV forward flow.
- Adjusting ECMO flows may help or may make things worse. For example, increasing ECMO flows will direct a higher proportion of venous return into the circuit, and will reduce systemic venous return through the pulmonary circulation. But this may increase afterload on the LV and exacerbate the situation further.
- Ensure proper positioning of access cannula in the right atrium. An inappropriately placed access cannula can result in inadequate drainage of the venous system and poor flows.
- Manage systemic volume overload. If systemic volume overload is contributing to LV distension, this should be addressed by aggressive diuresis or hemofiltration.
TTE and CXR confirmed placement of access cannula in the right atrium. ECMO flows were increased to 3.5L/min. He had been on CRRT due to AKI, with aggressive fluid removal and milrinone was commenced for inotropic support. Despite these measures, pulmonary oedema persisted.
Q6. What other options are available for LV decompression?
Many methods have been described for LV decompression or venting – here are some examples.
Intraaortic balloon pumps
- IABP reduce the LV afterload and thus potentially help LV ejection and emptying. Several non randomized studies have suggested the association of IABP and improved outcomes in VA ECMO.
Pigtail catheter in the LV
- The LV can be directly vented through a percutaneous pigtail catheter that is inserted directly into the LV via the aorta and aortic valve. The proximal end is joined to the access limb of the ECLS circuit and usually enters via the femoral vein. Several experimental animal models reported a significant reduction in LV preload, total energy and work when comparing pre- and post-cannula insertion. Results in humans are largely limited to case reports and series. Fumagalli and colleagues described draining blood from the LV via a percutaneously inserted transaortic cannula directly into the femoral artery with normalisation of left heart filling pressures and resolution of pulmonary oedema. Hong et al published a series of 7 adult patients with transaortic catheter venting. They showed that survivors had reductions in LV size and that no procedural complications occurred.
Surgical access cannula in the LV
- Surgical LV venting can be done via transapical cannulation under direct vision. This option is less attractive in settings where the chest is not already open. In such settings, decision for a centrally inserted vent should be weighed against the risk of bleeding complications due to systemic anticoagulation.
Percutaneous transseptal access cannula
- Percutaneous transseptal venting of the left heart can be achieved via blade balloon atrial septostomy and transseptal cannulation. Due to difficulties in achieving unrestricted left-to-right flow with septostomy, transseptal cannulation is preferred as it is thought to provide better left heart decompression. The disadvantages of transseptal venting include septal injury and possible left-to-right shunt formation. Furthermore, there is no direct LV unloading in the absence of significant mitral insufficiency.
An example is shown of a pigtail catheter inserted into the LV via the transaortic route. This catheter can be incorporated into the access cannula of the ECLS circuit (see diagram below)

Figure 3. ECMO circuit diagram showing how left ventricular “vent” can be incorporated in the circuit. In this diagram a transaortic catheter is used. Image from Hong et al, 2016. (Click image for source).
The pigtail catheter can be visualised on echo as well:
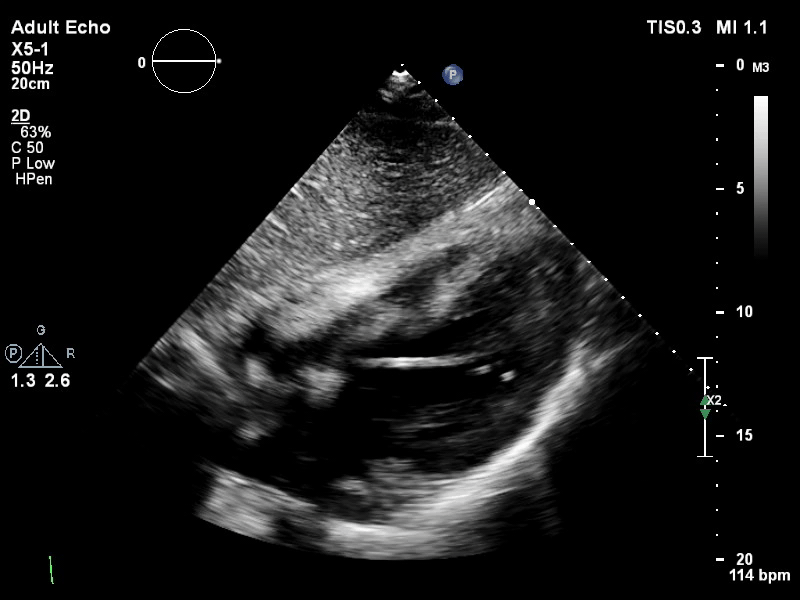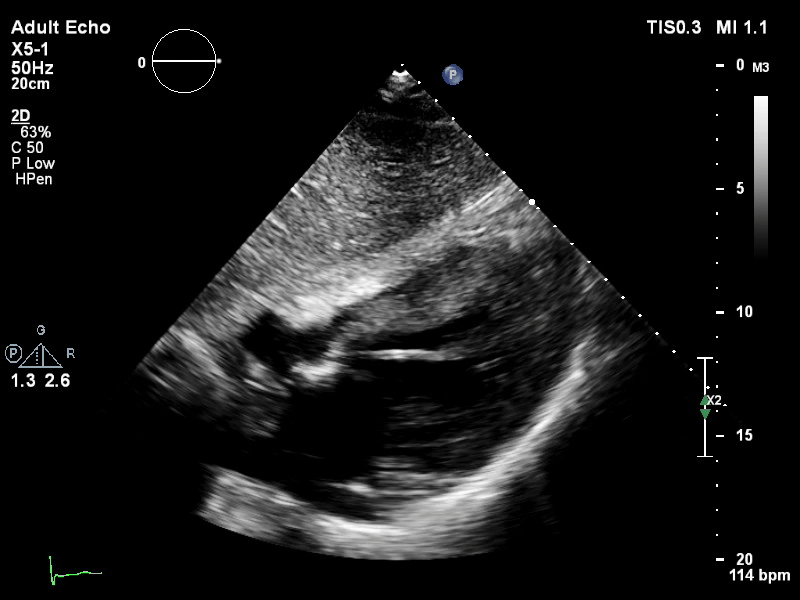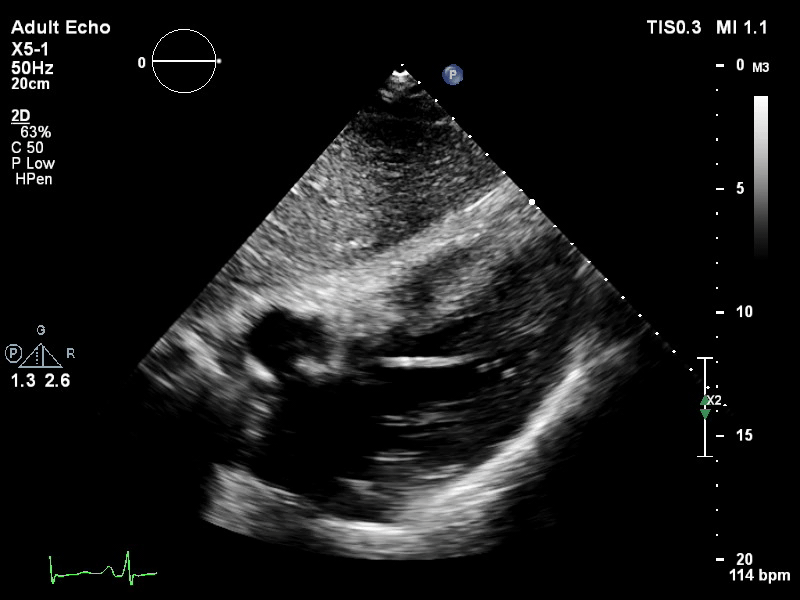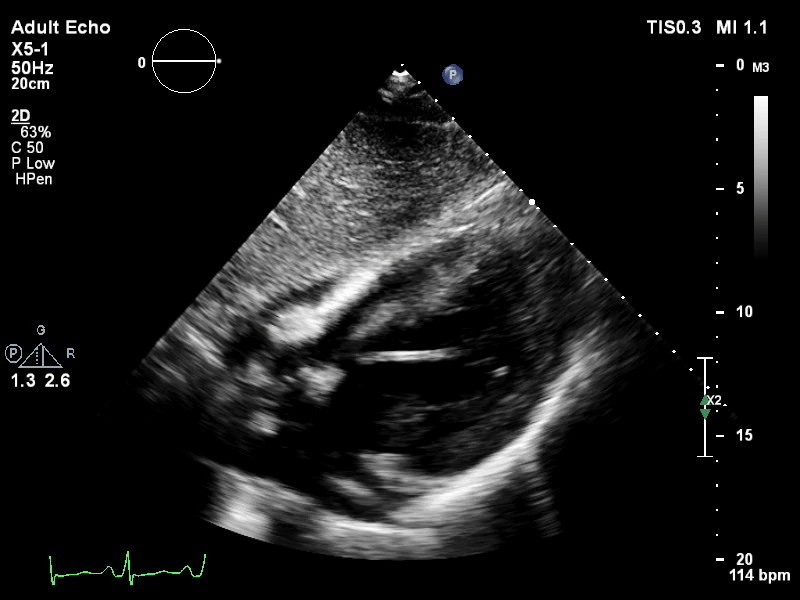
TTE subcostal view of distended left ventricle with transoaortic pigtail catheter in situ on VA ECMO.

TTE parasternal long axis view of distended left ventricle with transaortic pigtail catheter in situ on VA ECMO.
Q5. Discuss the likely outcomes for this patient?
There is no established method for assessing adequate LV decompression. Parameters which could be used include resolution of pulmonary oedema on chest x-ray and echocardiograhic indices such as aortic valve opening and left heart chamber dimensions.
Despite some promising methods for the decompression of the LV, many patients do not survive this condition.
At the Alfred ICU we have had cases of successful LV decompression with a pigtail catheter allowing successful bridging to LVAD insertion.
References
- Alkhouli M, Narins CR, Lehoux J, Knight PA, Waits B, Ling FS. Percutaneous Decompression of the Left Ventricle in Cardiogenic Shock Patients on Venoarterial Extracorporeal Membrane Oxygenation. J Card Surg. 2016;31(3):177-82. [pubmed]
- Baruteau AE, Barnetche T, Morin L, et al. Percutaneous balloon atrial septostomy on top of venoarterial extracorporeal membrane oxygenation results in safe and effective left heart decompression. Eur Heart J Acute Cardiovasc Care. 2018;7(1):70-79. [article]
- Fumagalli R, Bombino M, Borelli M, et al. Percutaneous bridge to heart transplantation by venoarterial ECMO and transaortic left ventricular venting. Int J Artif Organs. 2004;27(5):410-3. [article]
- Hong TH, Byun JH, Lee HM, et al. Initial Experience of Transaortic Catheter Venting in Patients with Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. ASAIO J. 2016;62(2):117-22. [article]
- Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015;65(19):e7-e26. [article]
- Soleimani B, Pae WE. Management of left ventricular distension during peripheral extracorporeal membrane oxygenation for cardiogenic shock. Perfusion. 2012;27(4):326-31. [article]
- Weymann A, Schmack B, Sabashnikov A, et al. Central extracorporeal life support with left ventricular decompression for the treatment of refractory cardiogenic shock and lung failure. J Cardiothorac Surg. 2014;9:60. [article]


Many a times faced this situation. Tried everything except transaortic pigtail but no positive outcome.